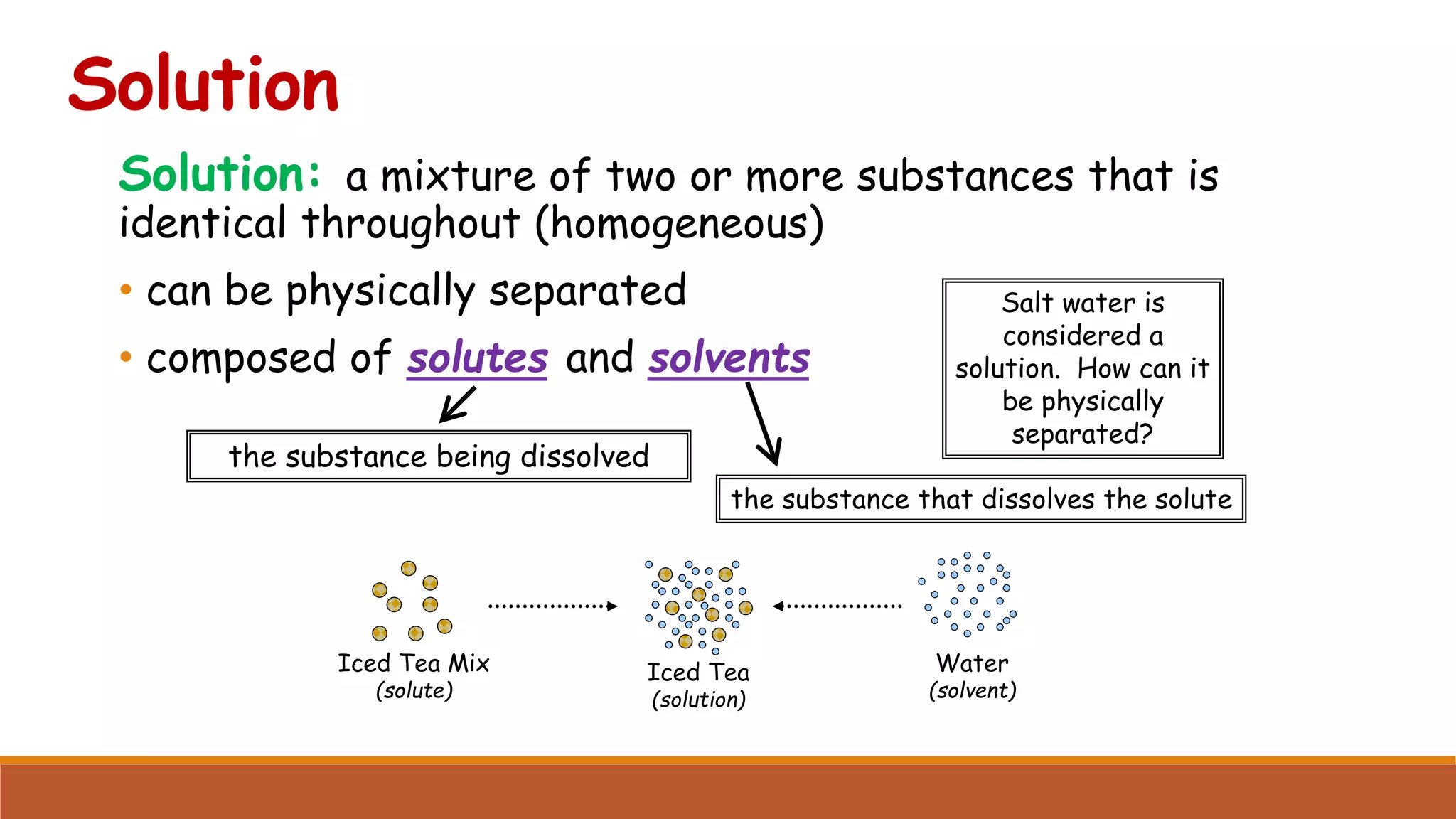
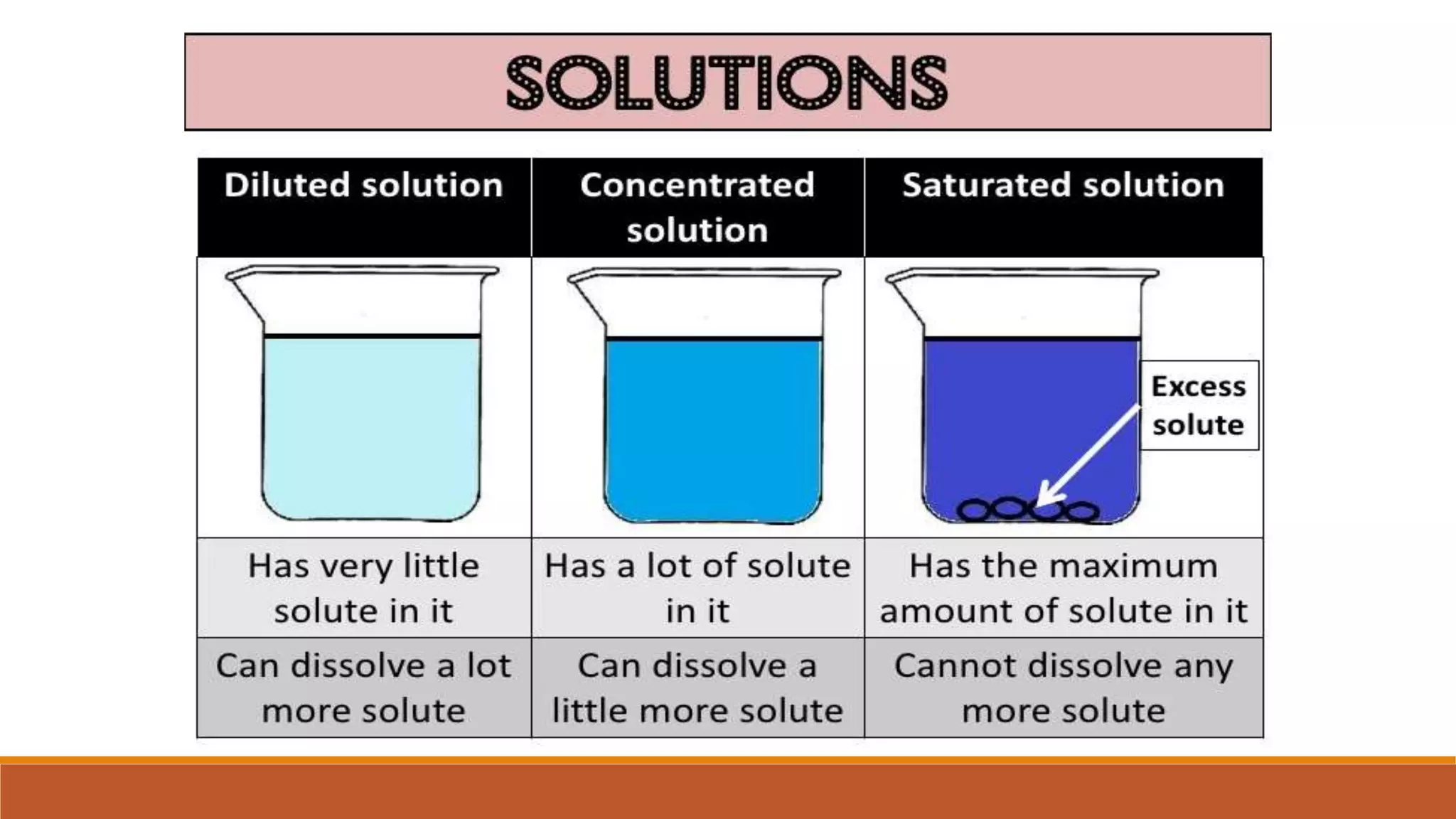
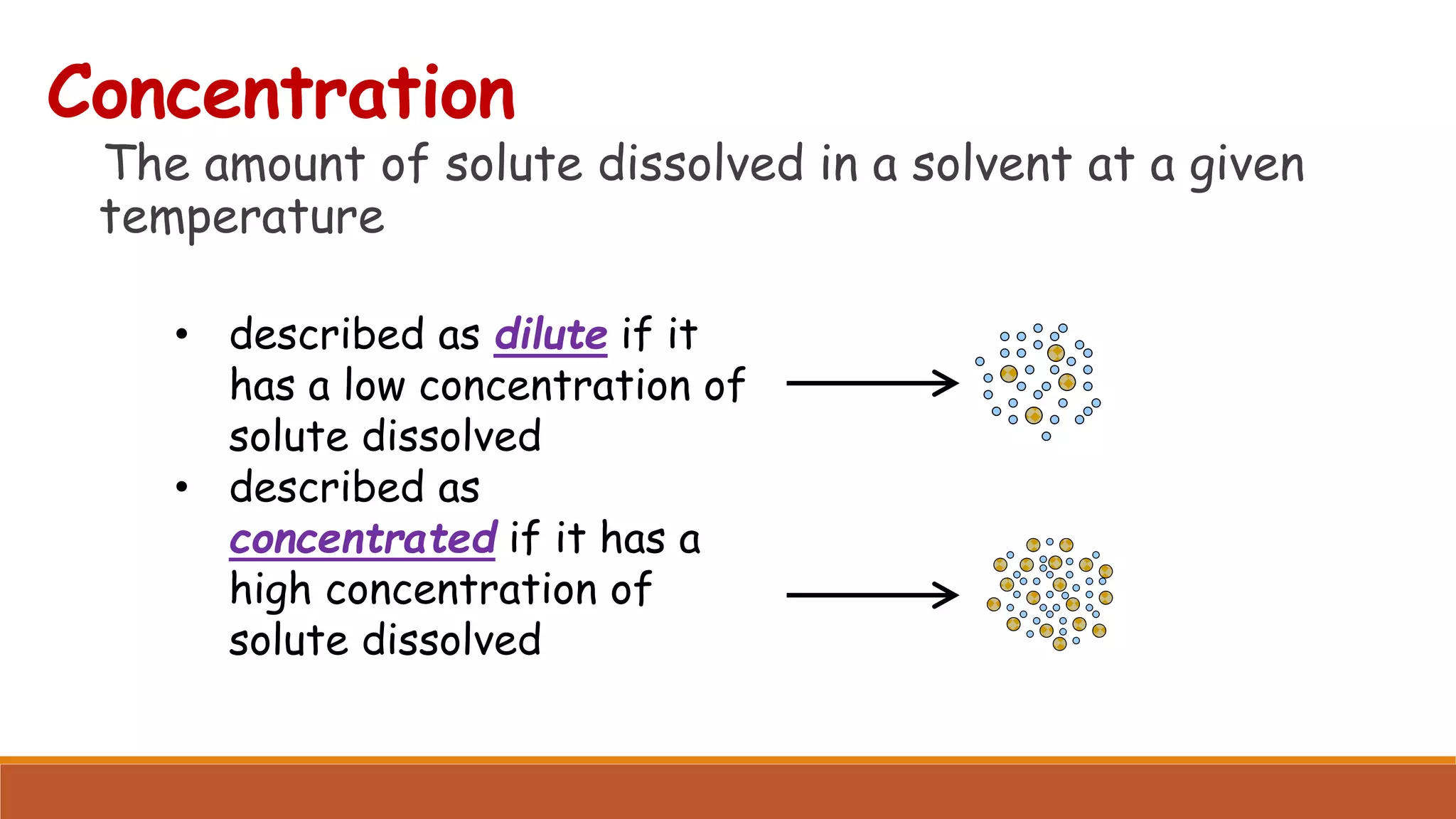
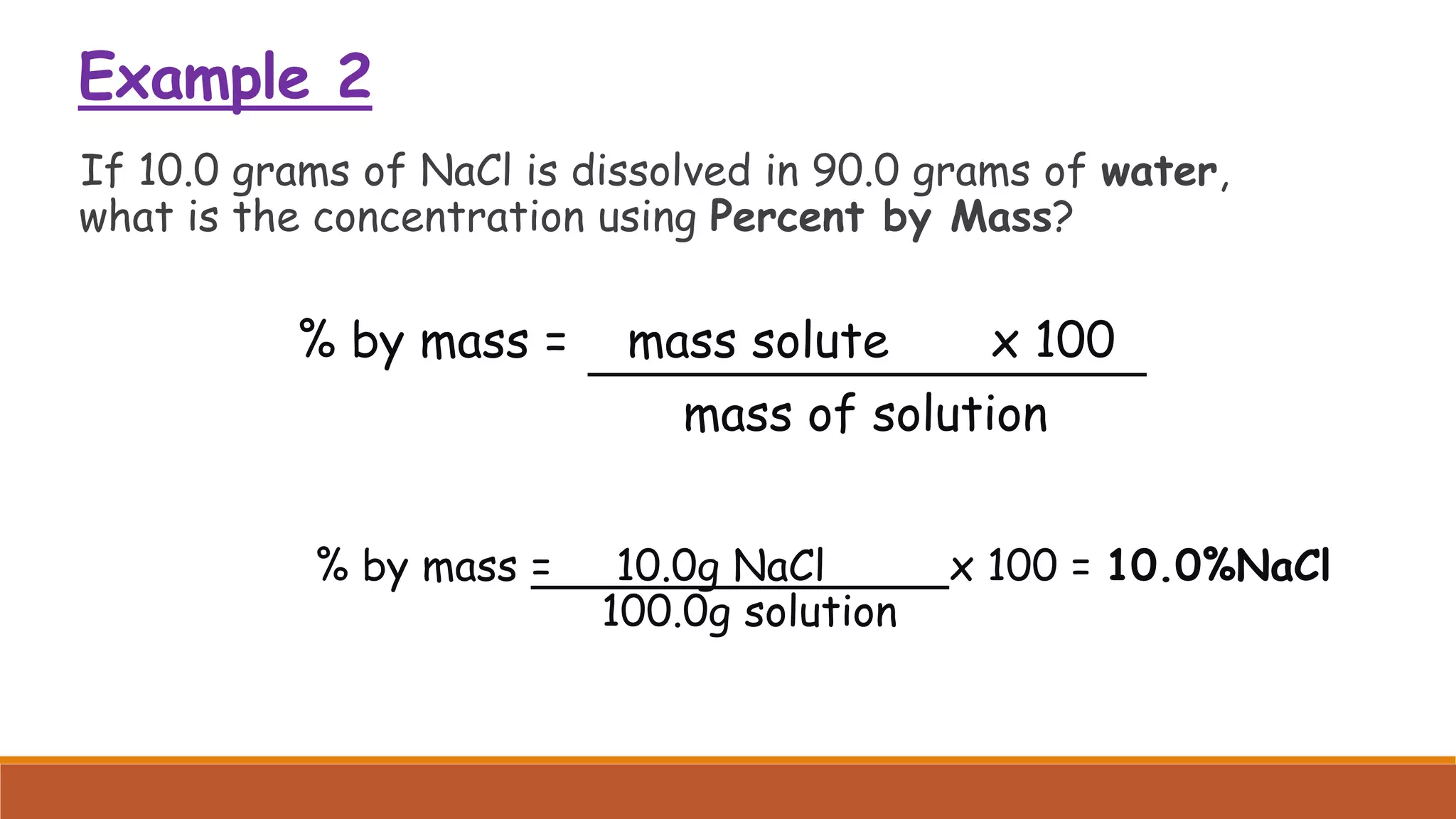
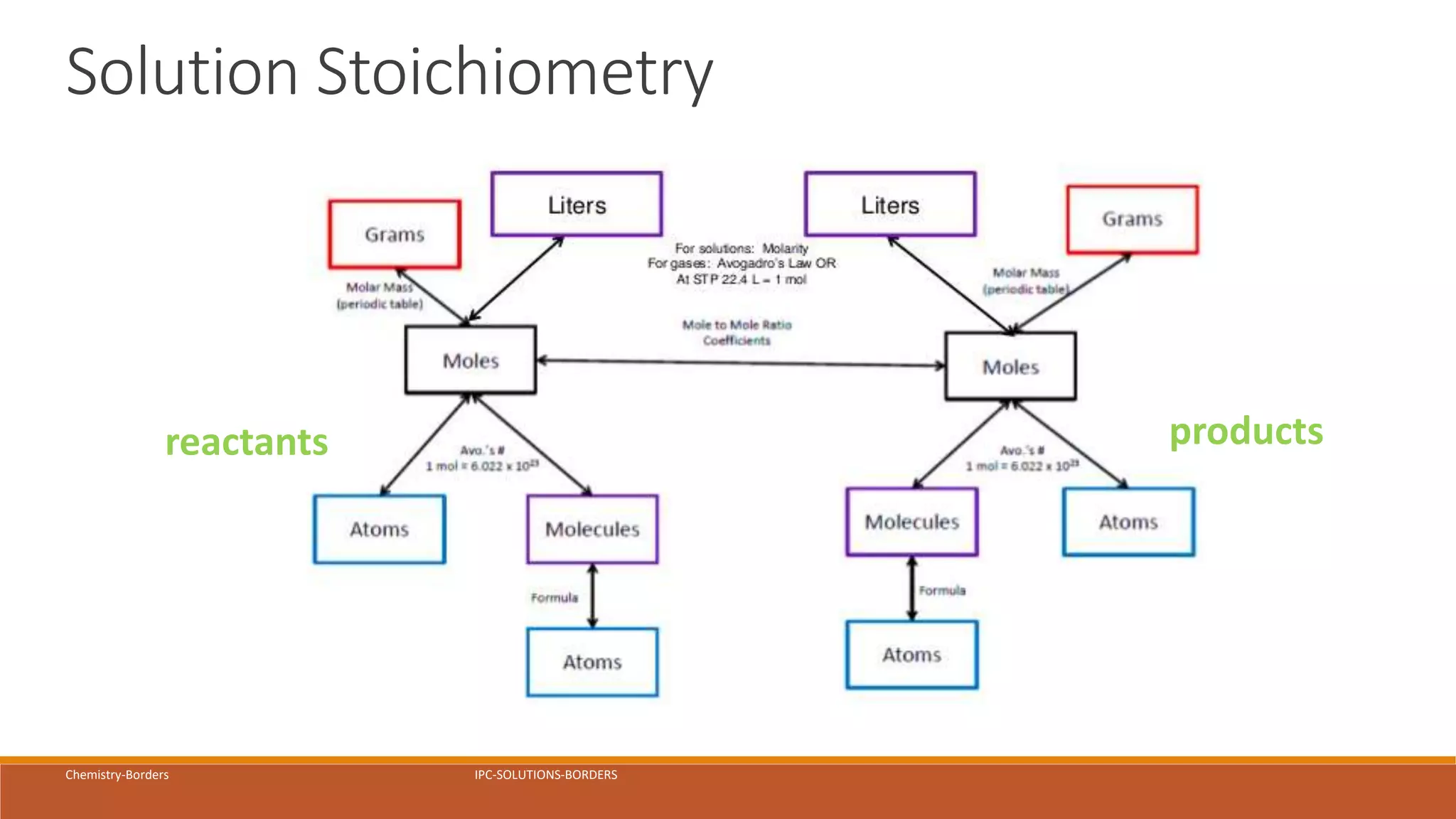
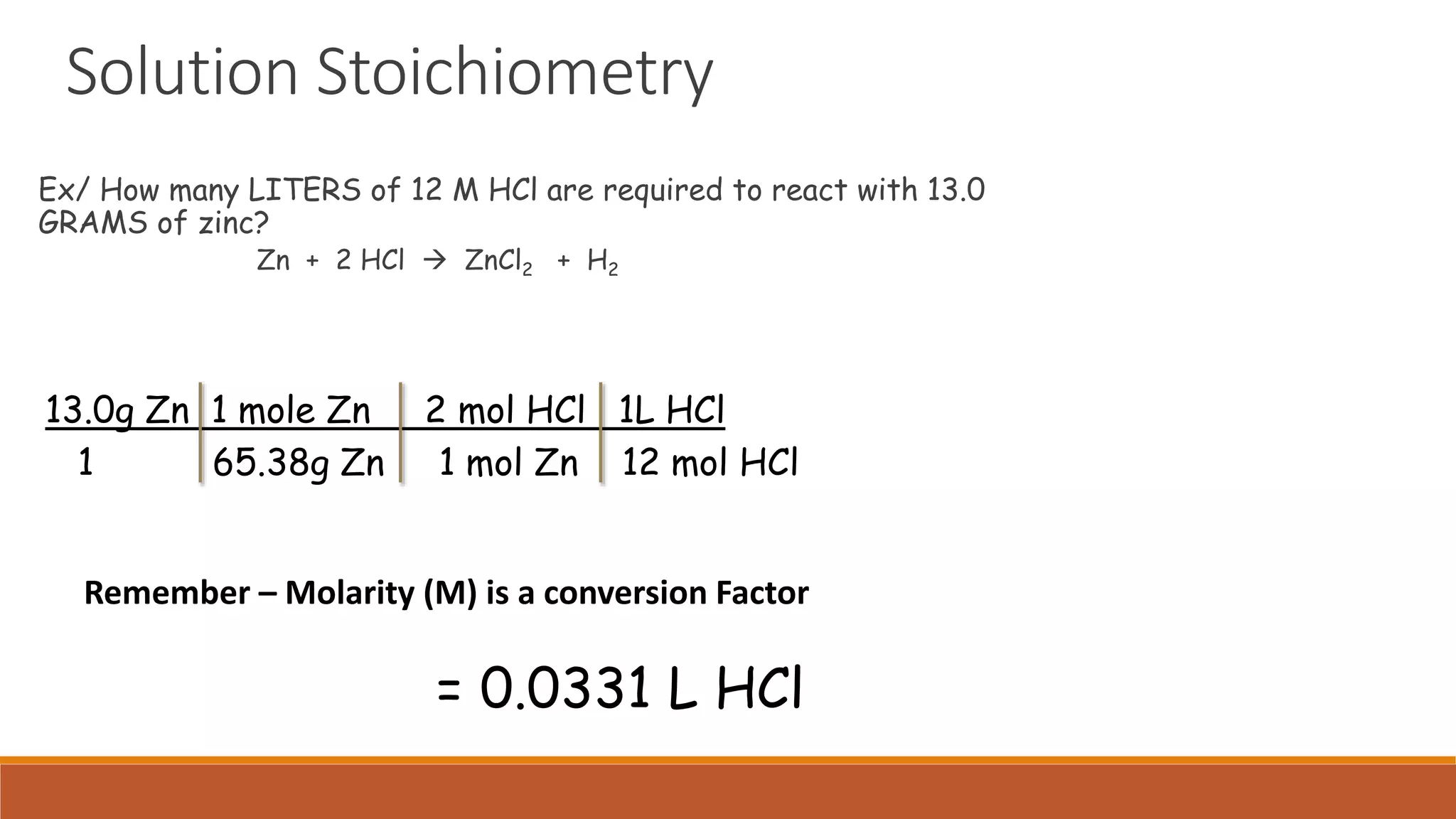
The document provides information on different ways of expressing the concentration of solutions, including percent by mass, mole fraction, molarity, molality, percent by volume, and parts per million (ppm). It discusses an activity where students are asked to mix a substance with water and observe whether the mixture is uniform or non-uniform. Finally, it defines key terms related to solution concentration such as solution, solute, solvent, concentration, solubility, miscible, and immiscible.