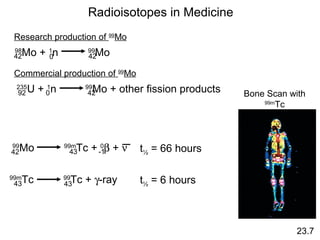
This document discusses nuclear chemistry and includes the following key points:
- Atomic number (Z) is the number of protons and mass number (A) is the number of protons + neutrons.
- Nuclear equations must balance mass number and atomic number.
- Radioactive decay occurs through alpha, beta, and positron emission or electron capture to achieve nuclear stability.
- The rate of radioactive decay follows first-order kinetics and half-life can be used for radioactive dating.








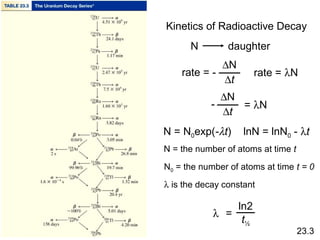
![Kinetics of Radioactive Decay
[N] = [N]0exp(-λt) ln[N] = ln[N]0 - λt
[N]
ln[N]
23.3](https://image.slidesharecdn.com/chapter23nuclearchemistry-150223191118-conversion-gate02/85/Chapter-23-nuclear_chemistry-13-320.jpg)


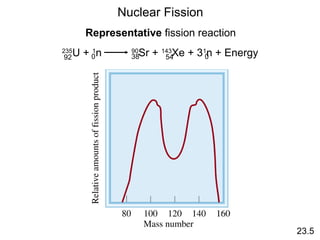
![Nuclear Fission
23.5
235
U + 1
n 90
Sr + 143
Xe + 31
n + Energy92 54380 0
Energy = [mass 235
U + mass n – (mass 90
Sr + mass 143
Xe + 3 x mass n )] x c2
Energy = 3.3 x 10-11
J per 235
U
= 2.0 x 1013
J per mole 235
U
Combustion of 1 ton of coal = 5 x 107
J](https://image.slidesharecdn.com/chapter23nuclearchemistry-150223191118-conversion-gate02/85/Chapter-23-nuclear_chemistry-17-320.jpg)