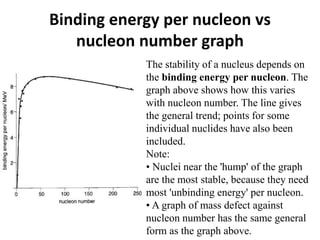
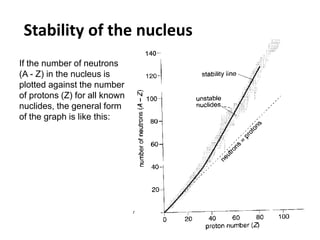
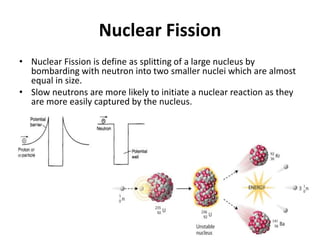
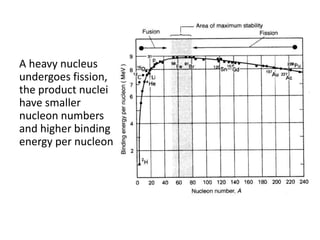
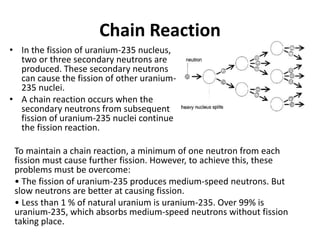
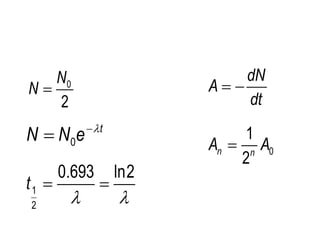Nuclear physics describes the structure and interactions of atomic nuclei. Rutherford discovered the nucleus through alpha scattering experiments. Protons and neutrons were later identified. Isotopes have the same number of protons but different numbers of neutrons. Mass defect and binding energy explain why atomic nuclei are more stable than separated nucleons. Radioactive decay occurs spontaneously at a rate proportional to the number of unstable nuclei. Exponential decay and half-life are described by the decay constant. Nuclear reactions conserve nucleon number and charge. Energy is released or absorbed through mass-energy equivalence. Fission and fusion occur under different conditions according to binding energy. Controlled fission in reactors uses moderation and feedback to sustain a chain reaction. Fusion






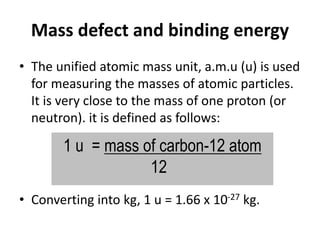
![Mass defect
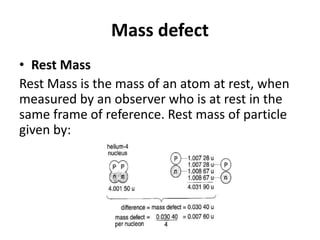
Mass Defect m can be concluded as follows:
m=[Zmp+(A-Z)mn]-MN
mp = mass of proton
mn = mass of neutron
MN = mass of nucleus (composite mass of nuclide)
Mass defect is the mass different between composite
mass of nuclide nucleon and the sum of it nucleons.](https://image.slidesharecdn.com/25-230717024427-8b6cd8c8/85/25-0-Nuclear-Physics-Sem-3-pptx-9-320.jpg)

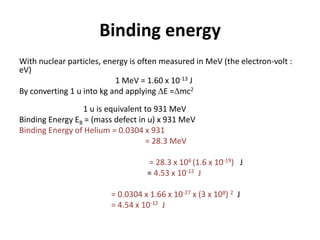
![Binding energy
• Binding energy is the nuclear energy required
to completely separate the nucleus of an atom
into its component (nucleons).
Binding Energy EB = (mass defect) x (c2)
= m c2
= [[Zmp+(A-Z)mn]-MN ] c2
Unit = Joule (J)](https://image.slidesharecdn.com/25-230717024427-8b6cd8c8/85/25-0-Nuclear-Physics-Sem-3-pptx-11-320.jpg)