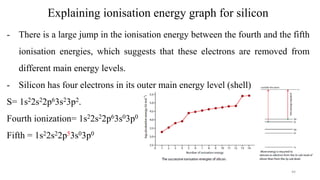
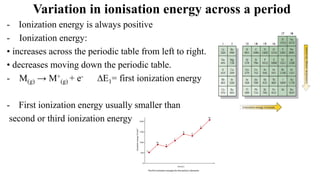
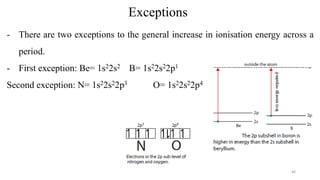
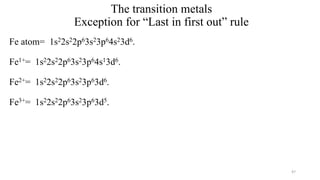
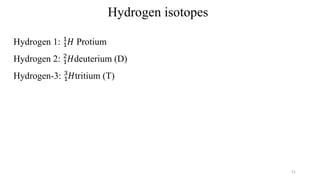
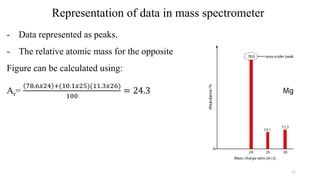
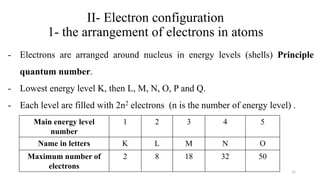
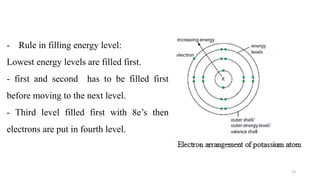
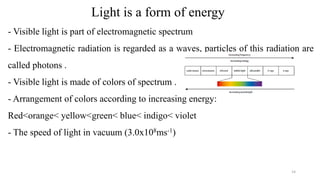
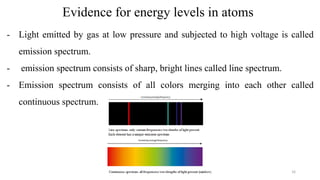
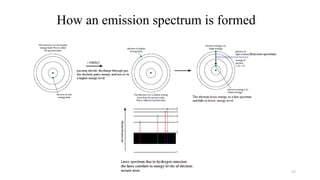
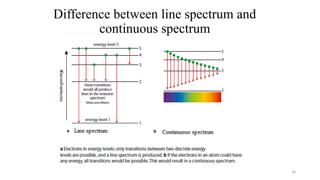
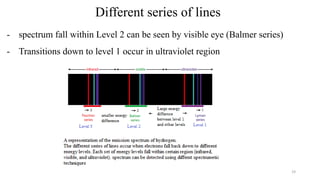
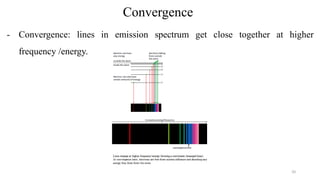
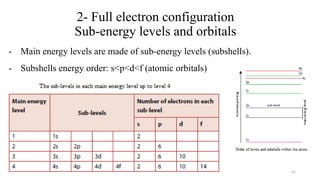
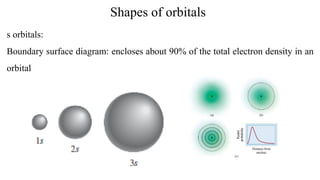
The document provides a comprehensive overview of atomic structure, detailing components such as the nucleus, isotopes, atomic mass, electron configuration, and quantum theory. It explains key concepts including ionization energy, the arrangement of electrons in energy levels, and the significance of mass spectroscopy in determining atomic and molecular masses. Additionally, it discusses historical figures in the development of atomic theory and the nature of light as it relates to atomic energy levels.



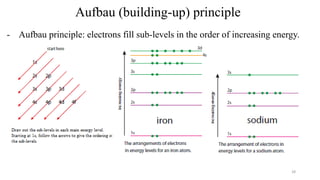
![Abbreviation for writing electronic
configuration
- Example: electronic configuration of germanium (Gr):
1s22s22p63s23p64s23d104p2 (Is abbreviated to [Ar]4s23d104p2)
- All atoms in the same group (vertical column) have the same outer shell
electronic configuration.
- Example: group 16 have outer shell electronic configuration: ns2np4 where n is
the period number
29](https://image.slidesharecdn.com/atomicstructureelectronicconfigurationib-171215124958/85/Atomic-structure-electronic-configuration-ib-29-320.jpg)


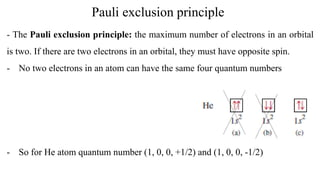
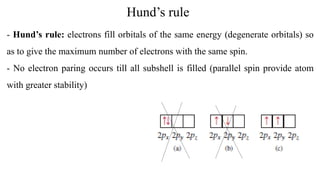
![Electronic configuration distribution
Exceptions:
24Cr: [Ar]3d54s1
29Cu: [Ar]3d104s1
35](https://image.slidesharecdn.com/atomicstructureelectronicconfigurationib-171215124958/85/Atomic-structure-electronic-configuration-ib-35-320.jpg)