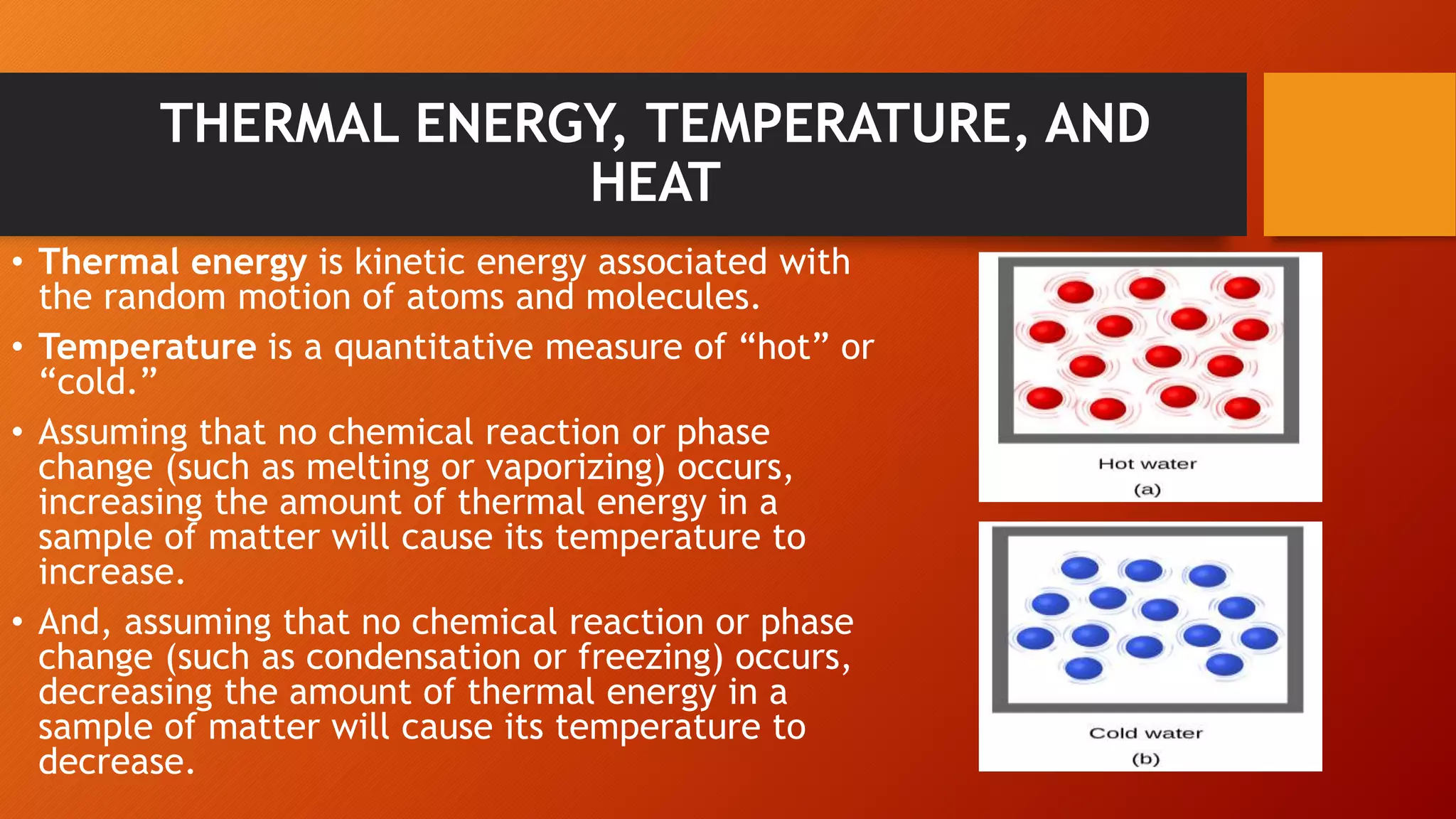
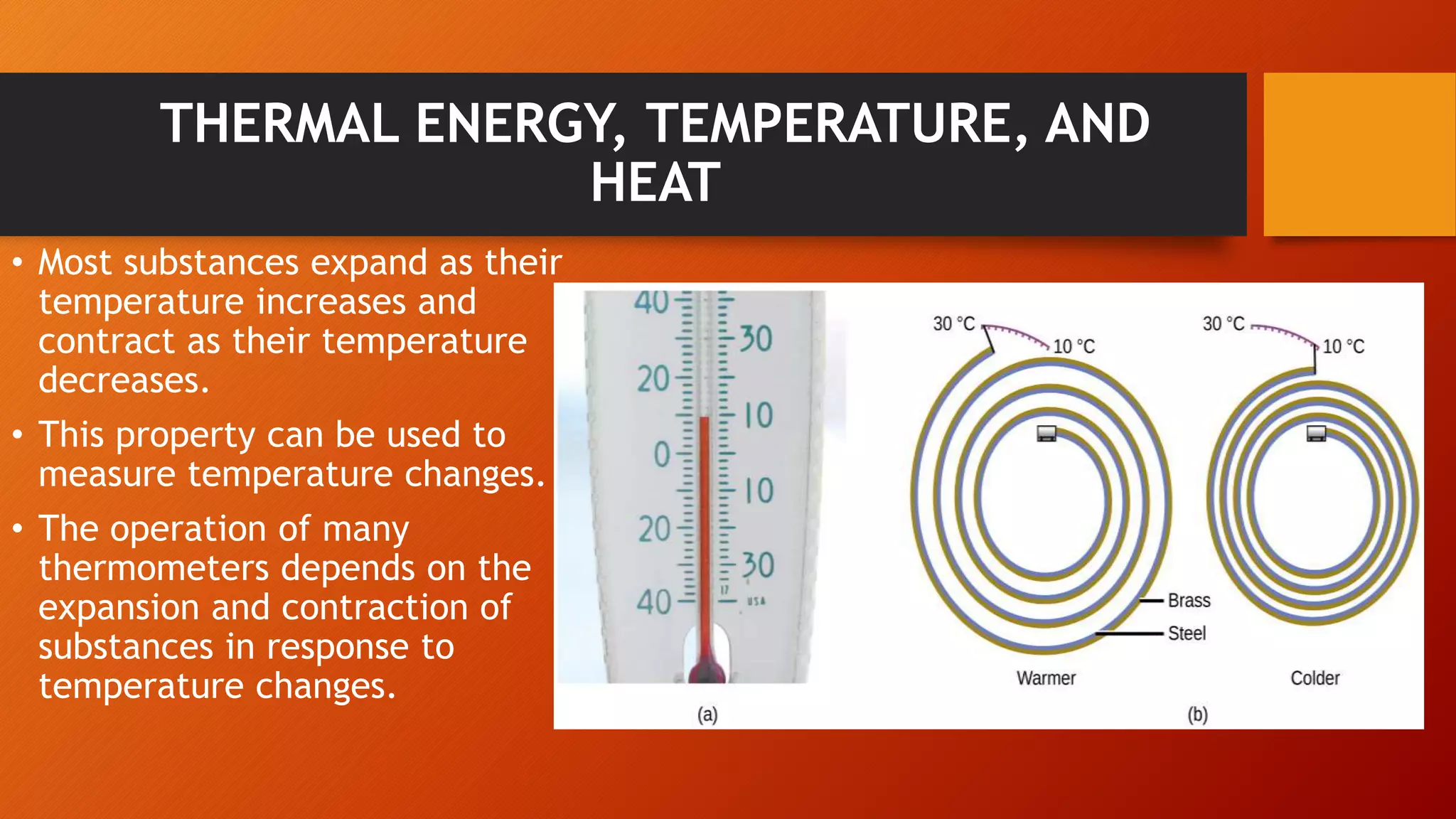
The document discusses key concepts in thermochemistry including:
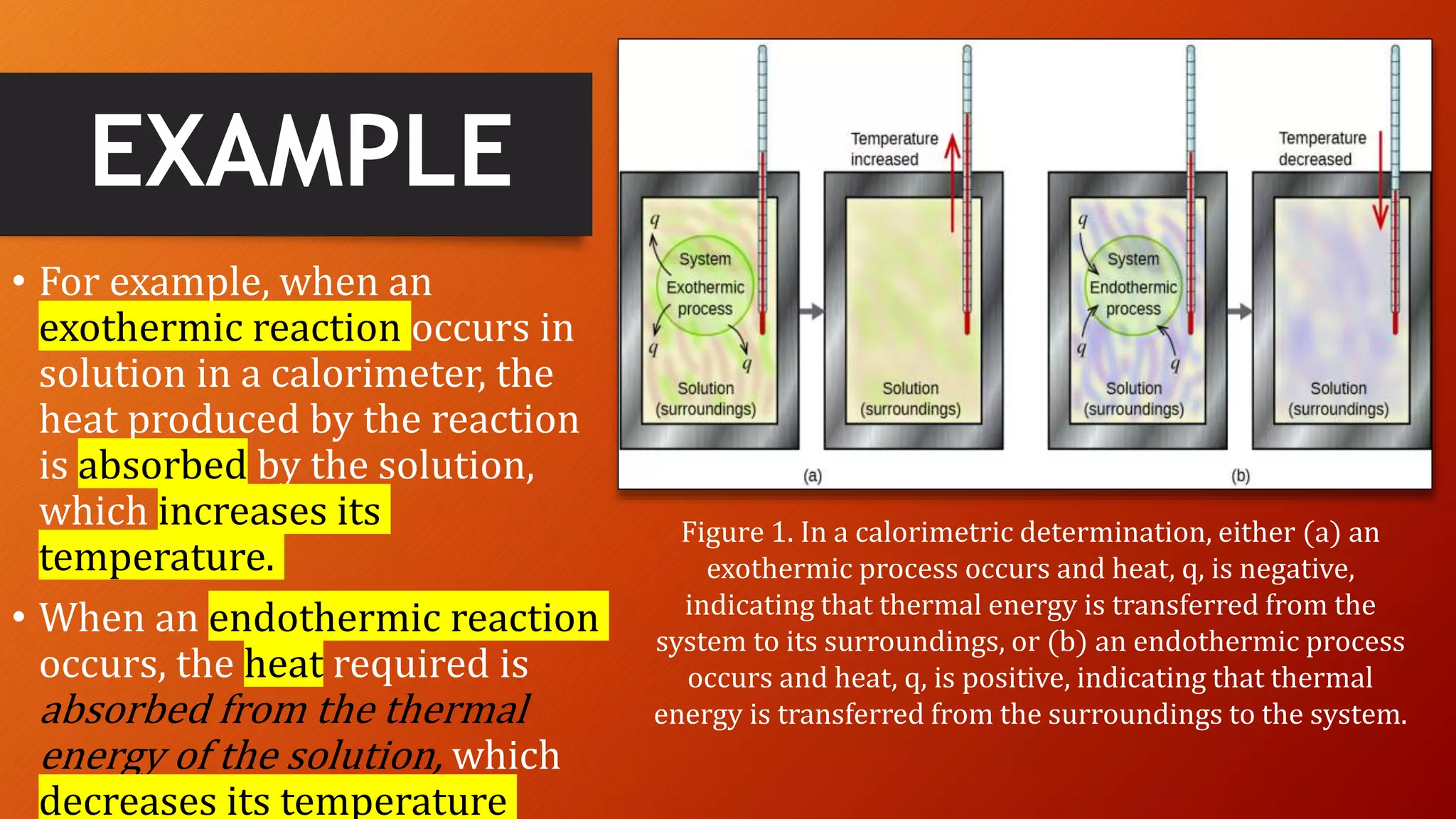
- Exothermic and endothermic processes that release or absorb heat during chemical reactions.
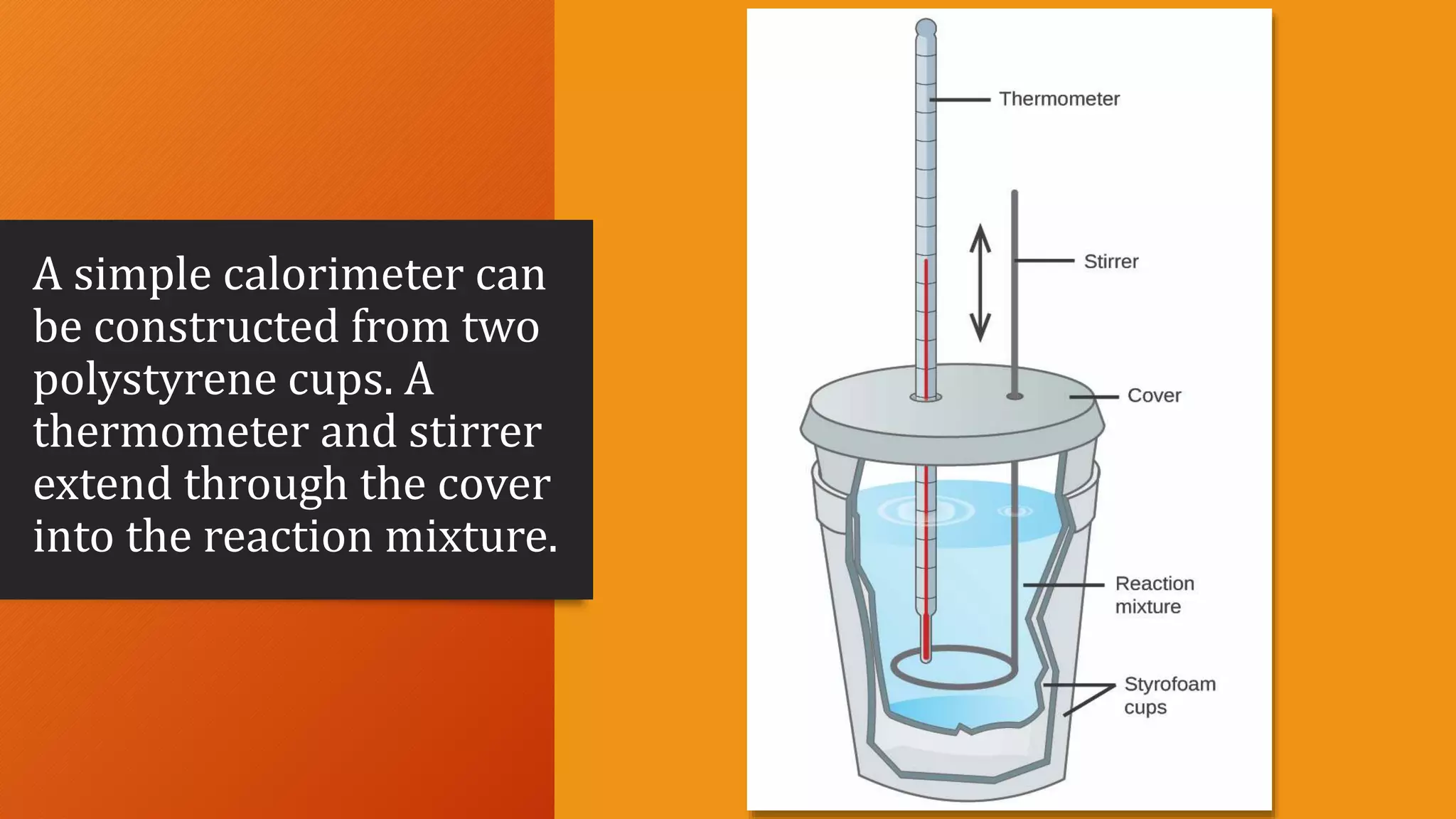
- Calorimetry, the process of measuring heat changes using devices called calorimeters.
- The first law of thermodynamics, also known as the law of conservation of energy, which states that energy cannot be created or destroyed, only changed from one form to another.
- Enthalpy, the heat content of a system, and how thermochemical equations are used to express energy changes in chemical reactions.
- Standard enthalpy of formation and reaction, and Hess's law which describes how the energy change in an overall reaction is the same regardless of