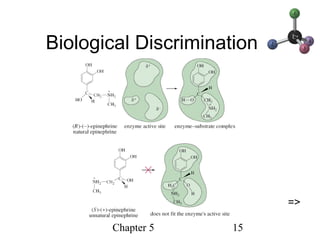
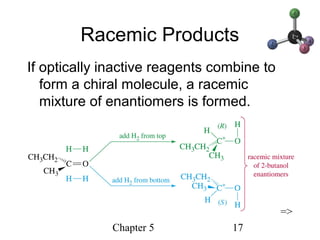
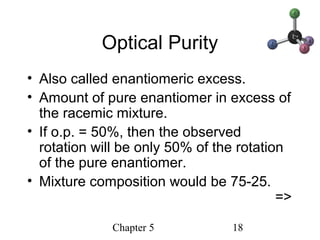
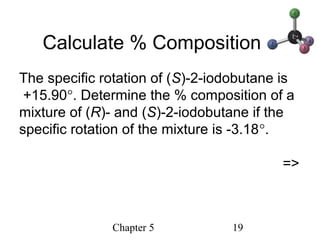
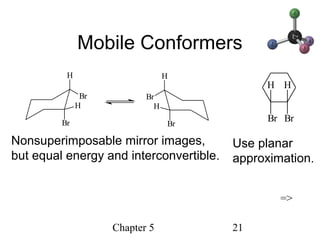
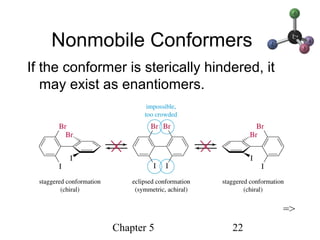
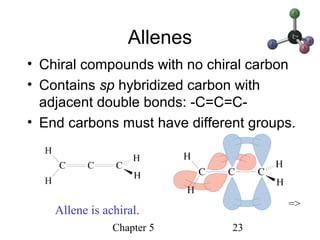
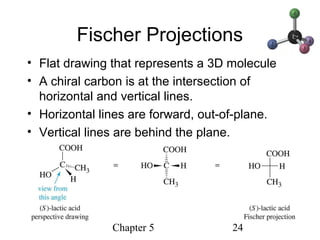
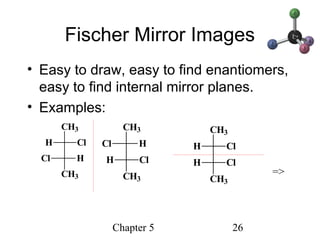
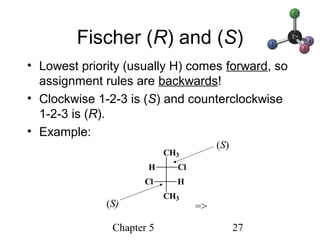
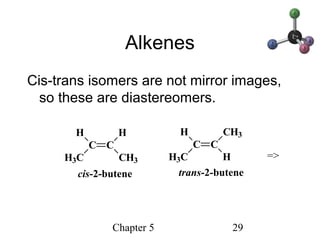
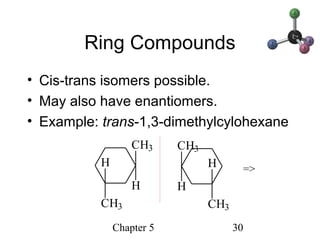
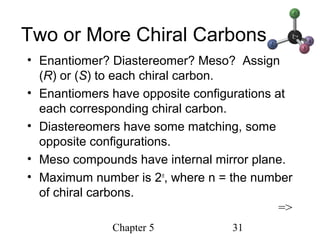
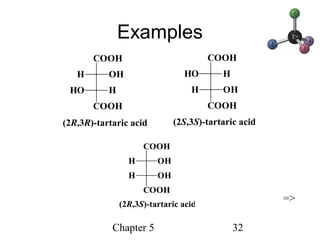
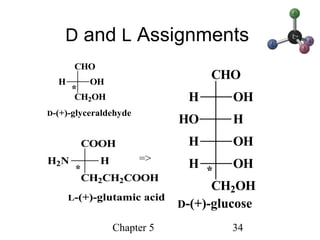
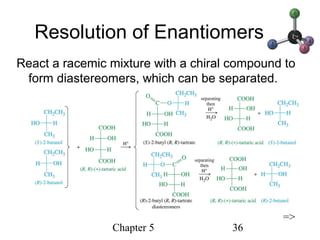
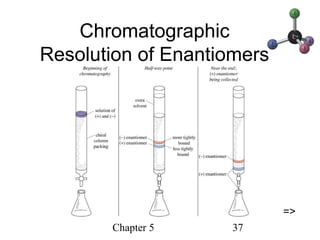
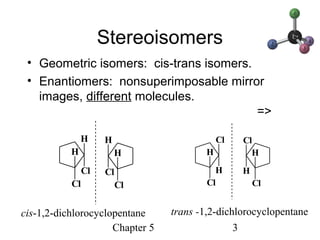
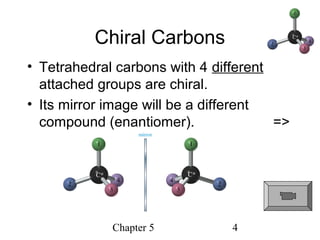
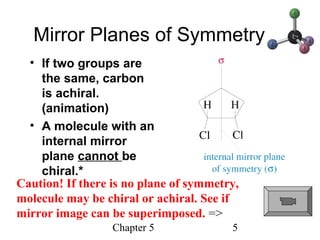
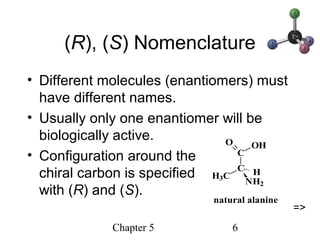
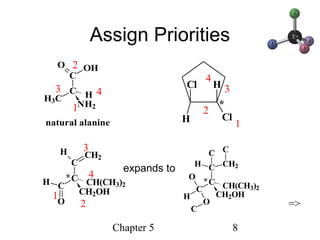
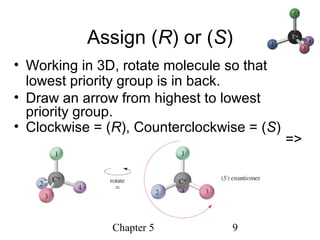
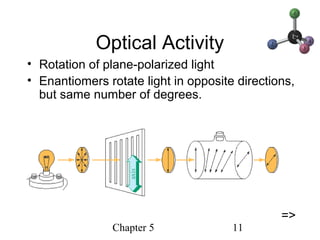
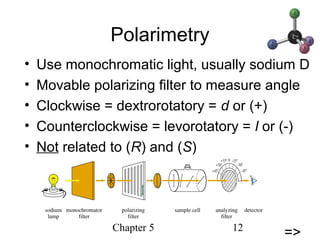
This chapter discusses stereochemistry and chirality. It defines stereoisomers such as enantiomers, which are nonsuperimposable mirror images, and diastereomers, which are not mirror images. Chiral carbons have four different groups and exist as enantiomers. Enantiomers have identical properties except for how they interact with other chiral molecules and rotate plane-polarized light in opposite directions. Methods to determine chirality such as assigning R/S configurations and using Fischer projections are covered. The chapter also discusses resolving enantiomers through formation of diastereomers.



![Chapter 5 13
Specific Rotation
Observed rotation depends on the length
of the cell and concentration, as well as
the strength of optical activity,
temperature, and wavelength of light.
[α] = α (observed)
c • l
c is concentration in g/mL
l is length of path in decimeters.
=>](https://image.slidesharecdn.com/stereochemistry-150913062253-lva1-app6892/85/Stereochemistry-13-320.jpg)
![Chapter 5 14
Calculate [α]D
• A 1.00-g sample is dissolved in 20.0 mL
ethanol. 5.00 mL of this solution is
placed in a 20.0-cm polarimeter tube at
25°C. The observed rotation is 1.25°
counterclockwise.
=>](https://image.slidesharecdn.com/stereochemistry-150913062253-lva1-app6892/85/Stereochemistry-14-320.jpg)