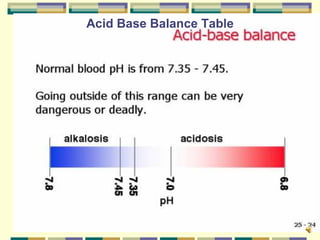
This document provides an overview of acid-base imbalances, including the key components of pH, PCO2, and base excess. It discusses respiratory and metabolic acid-base disorders, how the body controls acid-base balance through buffers, lungs, and kidneys, and the symptoms and treatments of common acid-base imbalances like metabolic acidosis, metabolic alkalosis, respiratory acidosis, and respiratory alkalosis.