This document discusses acid-base disorders and provides information on respiratory acidosis and metabolic alkalosis.
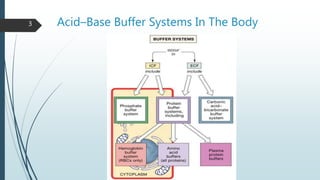
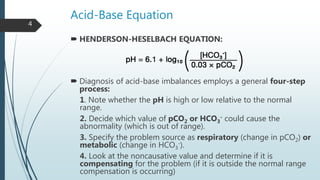
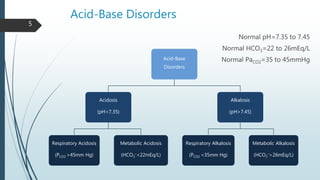
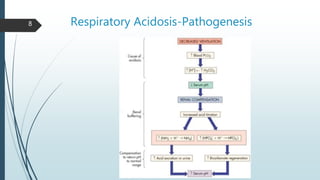
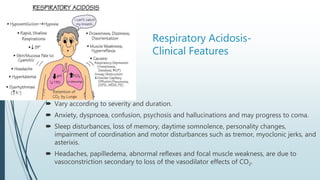
It begins with an overview of acid-base buffer systems in the body and the Henderson-Hasselbalch equation used to diagnose acid-base imbalances. It then discusses respiratory acidosis, defining it as a decreased pH and elevated pCO2. Causes include lung diseases that impair ventilation. It increases H+ excretion and HCO3- reabsorption as compensation.
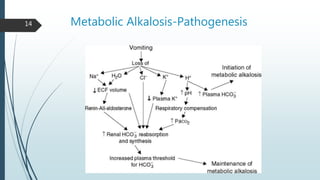
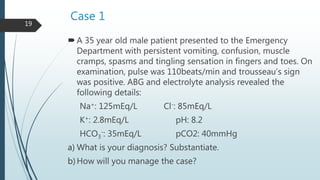
Metabolic alkalosis is then covered, defined as an increased pH and HCO3- concentration. Causes include vomiting, diuretic use, and mineralocorticoid excess. It decreases ventilation as compensation. Two