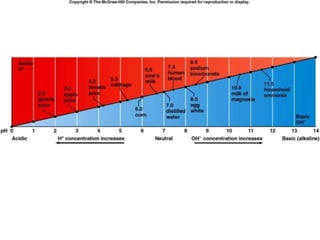
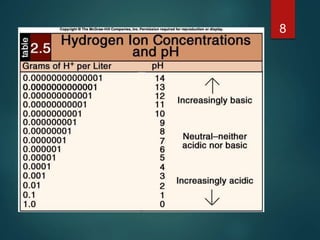
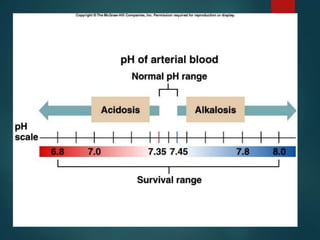
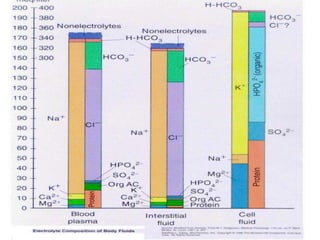
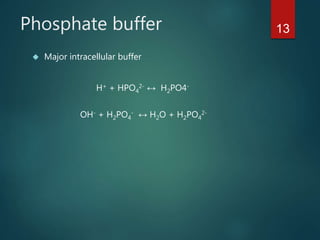
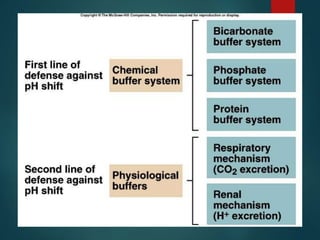
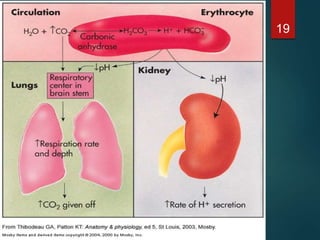
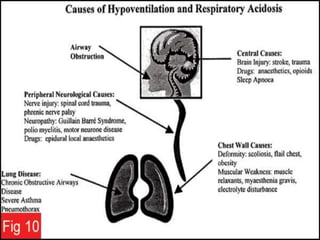
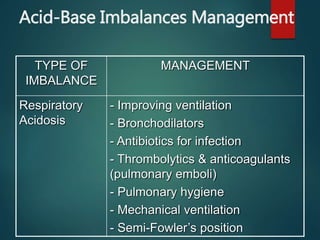
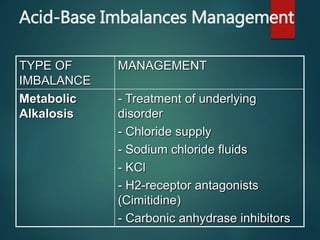
The document discusses the physiology and treatment of acid-base disorders, detailing the causes, signs, symptoms, and management strategies for conditions like acidosis and alkalosis. It emphasizes the importance of understanding arterial blood gas values for patient assessment and outlines the roles of respiratory and renal systems in maintaining acid-base balance. The document also categorizes various acid-base imbalances and their respective compensatory mechanisms.