This document provides an overview of acid-base balance and disorders. It discusses the major buffer system involving carbonic acid and bicarbonate, and how the lungs and kidneys work to maintain acid-base balance. Various acid-base disorders are described including their primary events, compensatory responses, and interpretations based on blood parameters such as bicarbonate, PCO2, and anion gap.




![Who do we express [H+]? Direct as [H+] which give H+ concentration in mol/L or Eq/L Indirect as PH: PH=Log10 1/[ H+]=-Log 10[H+] ***so PH + [H+] are inversely related *** ****The range of [H+] that is compatible with life is 20-126 nEq/L which is equivalent to PH range of 7.7-6.9 [H=] PH 128 6.9 101 7.0 80 7.1 64 7.2 50 7.3 40 7.4 32 7.5 25 7.6 20 7.70](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-5-320.jpg)


![Henderson –Hasselbalch equation CO2 ↔ CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3- Body use this equation to control PH At equilibrium there are approximately 500mmol of CO2 for every 1mmol of H2CO3. 4000mmol of H2CO3 for every 1mmol of H+. Using H-H equation: PH=PK+log [H2CO3] \ [HCO3]….. [H+] =24 X PCO2/ [HCO3]](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-8-320.jpg)
![Advantage of H-H equation [H+] is determined by ratio of PCO2 & [HCO3-]. It is a useful mean to verify the accuracy of the laboratory data by calculating one of the variable from the other two.](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-9-320.jpg)


![Total CO2 [total CO2]= [ HCO3 -] +( S.PCO2) S : solubility constant for CO2 in plasma = 0.03mmol /L / mmHg Dissolved CO2 = S.PCO2 = 0.03 xPCO2 at PCO2 = 40mmHg , dissolved CO2 = 1.2mmol / L ***total CO2 in clinical laboratory is a measure of [HCO3] *** total CO2 gives little information about functional status of the lungs](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-12-320.jpg)


![BODY BUFFER SYSTEM Buffer : is a solution consisting of a weak acid + its conjugate base * * * * * Buffering is the primary mean by which large changes in [H+] are minimized . The most effective buffers are : H2CO3, H2PO4, Plasma protein( Hb)](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-15-320.jpg)
![HCO3- BUFFER SYSTEM we use HCO3- buffer system to determined acid base status depending on the isohydric -principle which states that all buffer pairs are in equilibrium with the same [H+].](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-16-320.jpg)



![Base excess (BE) It refers to the change in the concentration of buffer base (BB) [BB] = [ HCO3-]+[Protein-] +[ Hb / HbO2] = 48 mEq /L BE. Associated with abnormality in [HCO3] so it is influenced by metabolic process M. alkalosis associated with BE.> +5 M. acidosis associated with BE.< -5](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-20-320.jpg)
![Anion gap AG = ( [Na+] + [K+]) – ( [HCO3-] + [Cl-]) Normal AG =12 ± 4 m Eq\L why? Low of electro-neutrality](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-21-320.jpg)
![Low of electro-neutrality In any solution, positive charges = negative charges In serum -> [ cations ] = [ anions ], If we measured all cations + anions We measured every cations & only fraction of anions True if but](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-22-320.jpg)





![Osmolar Gap Refers to the disparity between the measured and the calculated serum osmolarity . =2[Na+] + (BUN/2,8) +(glucose/18) It is good screen for toxins as circulatory toxins ↑ measured osmolarity but not the calculated](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-28-320.jpg)


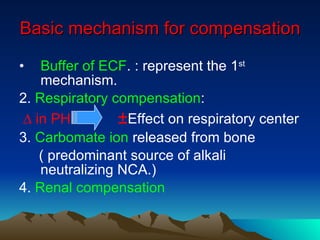

![Acute Respiratory Acidosis ***In pure acute Resp. acidosis , [HCO3-] should not exceed > 30mEq/l *** PCO2 ↑ by 10 mmHg -> ↑ HCO3- by 1 mEq /l ∆ [H+] = 0.8 ∆ PCO2](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-34-320.jpg)
![Chronic Respiratory Acidosis PCO2 ↑ by 10 mmHg -> ↑ HCO3 by 3.5 mEq/l ∆ [H+] = 0.3 X ∆ PCO2](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-35-320.jpg)


![Acute Respiratory Alkalosis / compensation *** Acute ↓ pco2-> rapid ↓ [HCO3-] within minutes / independent of any renal compensation (Buffer system) ↓ PCO2 by 10 mmHg -> ↓ HCO3- by 2 mEq/l ∆ [H+] = 0.8 X ∆PCO2](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-38-320.jpg)
![Chronic Respiratory Alkalosis / compensation ↓ PCO2 by 10 mmHg ->↓HCO3- by 5 mEq/l *** [H+] = 0.17 X ∆PCO2 ∆](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-39-320.jpg)

![Metabolic Acidosis ** Produced by any process that ↓ [HCO3] either as HCO3- loss, or via retention of NCA. That cannot be excreted by lungs](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-41-320.jpg)
![Metabolic Acidosis / Compensation ↓ [HCO3] by 1 mEq/l ->↓ PCO2 by 1.0_1.3 mmHg Example: Patient with CRF.& [HCO3]=16 PCO2 compensation range ->29.6_32mmHg If PCO2 = 24->->coexistent Resp. Alkalosis If PCO2 = 40 24->->coexistent Resp. Acidosis](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-42-320.jpg)


![Metabolic Alkalosis Produced by any process that ↑[HCO3] in plasma It is clinically useful to be divided into 2 types : 1. Chloride responsive 2. Chloride resistant ****** depending on urinary [ Cl- ] ****](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-45-320.jpg)
![Metabolic Alkalosis/ Compensation Highly variable ↑ [HCO3-] by 1 mEq/l -> ↑ PCO2 by 0.6_0.7 mmHg *** Any PCO2 exceeding 55 mmHg -> coexistence primary Resp. acidosis ***](https://image.slidesharecdn.com/cdocumentsandsettingssccdesktopacidbasebalance-090423162103-phpapp01/85/Acid-base-balance-46-320.jpg)