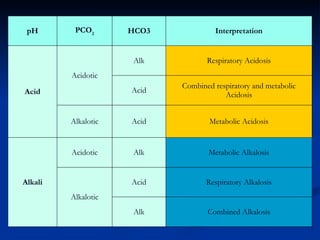
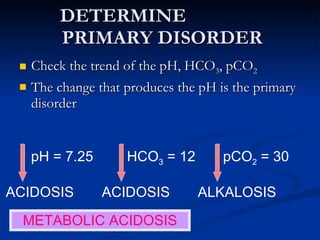
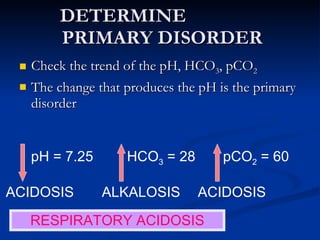
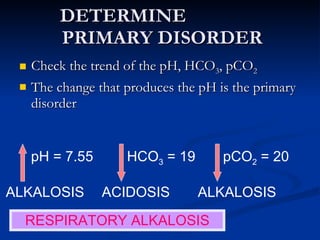
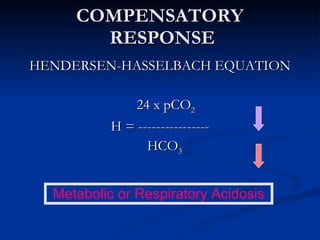
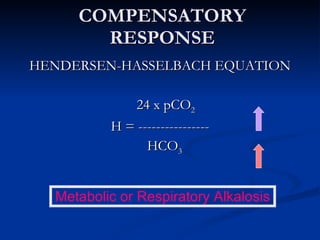
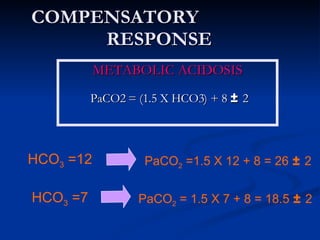
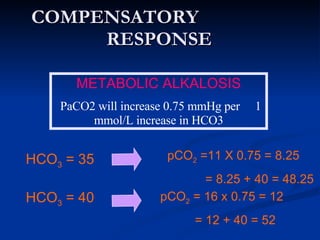
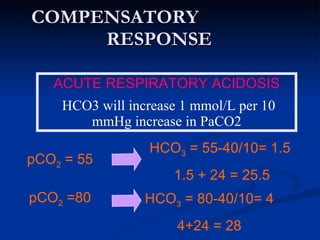
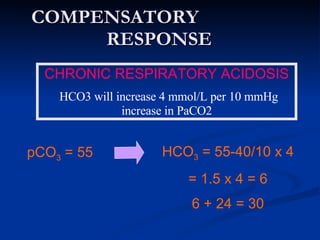
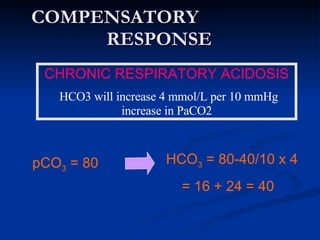
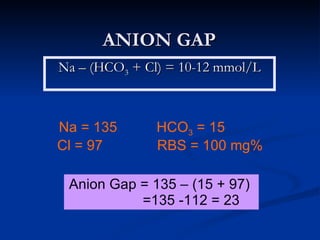
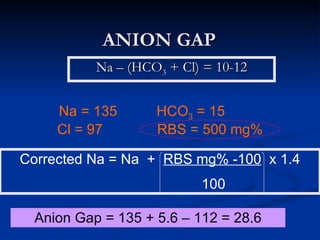
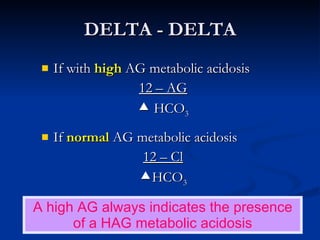
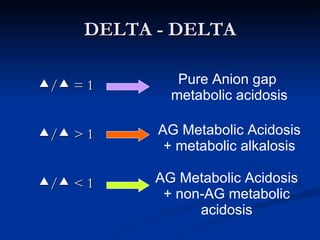
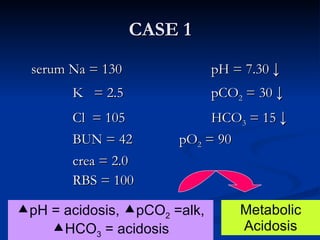
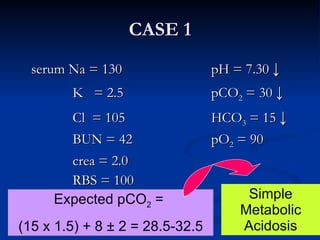
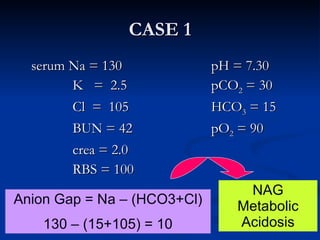
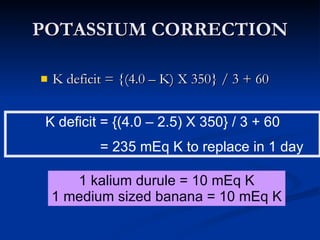
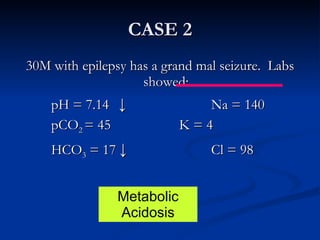
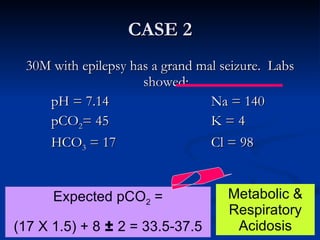
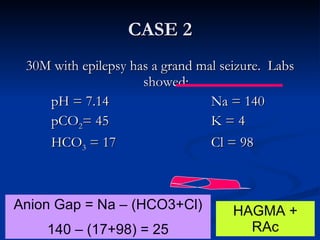
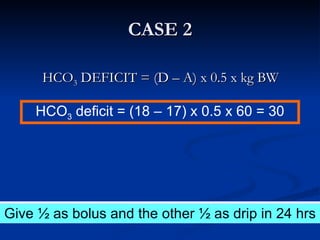
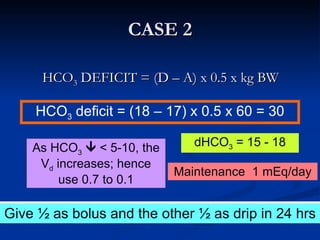
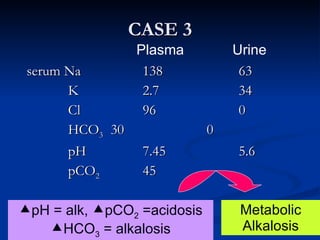
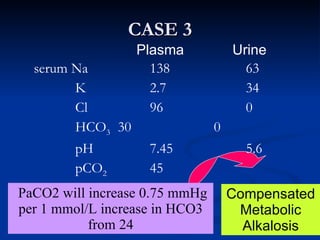
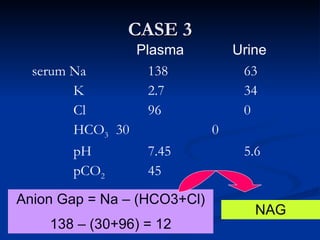
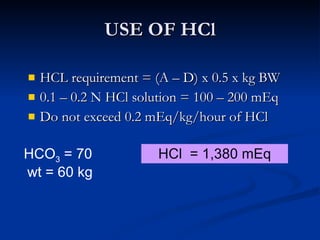
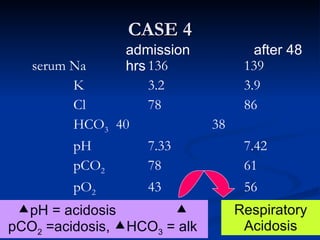
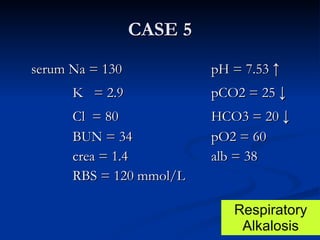
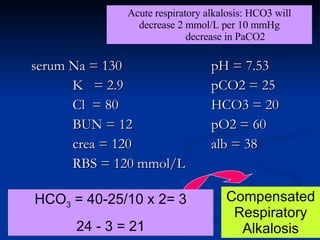
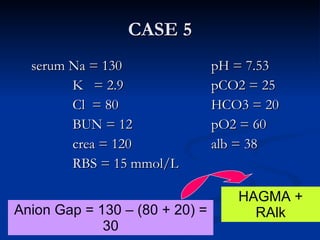
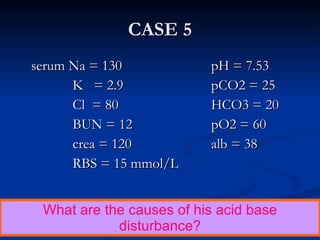
This document discusses acid-base disorders and interpretation of arterial blood gases (ABGs). It defines acidosis and alkalosis, and describes respiratory and metabolic causes. Simple and mixed acid-base disorders are explained. Compensation by the lungs and kidneys in response to primary disorders is discussed. A stepwise approach to ABG interpretation is provided, including determining the primary disorder, checking for compensation, calculating the anion gap, and identifying specific etiologies. Characteristics of simple acid-base disturbances and combined disorders are summarized.



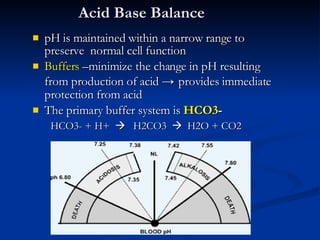


![Characteristics of the simple acid-base disturbances [HCO3-] Pco2 Respiratory alkalosis [HCO3-] Pco2 Respiratory acidosis Pco2 [HCO3-] Metabolic alkalosis Pco2 [HCO3-] Metabolic acidosis Compensated response Primary Primary pH Disorder](https://image.slidesharecdn.com/abg-chh-1218468866593910-9/85/ARTERIAL-BLOOD-GAS-INTERPRETATION-8-320.jpg)