The document discusses acid-base balance and arterial blood gas analysis. It provides details on:
1) How arterial blood gas analysis assesses oxygenation, ventilation, and acid-base status to diagnose acid-base imbalances.
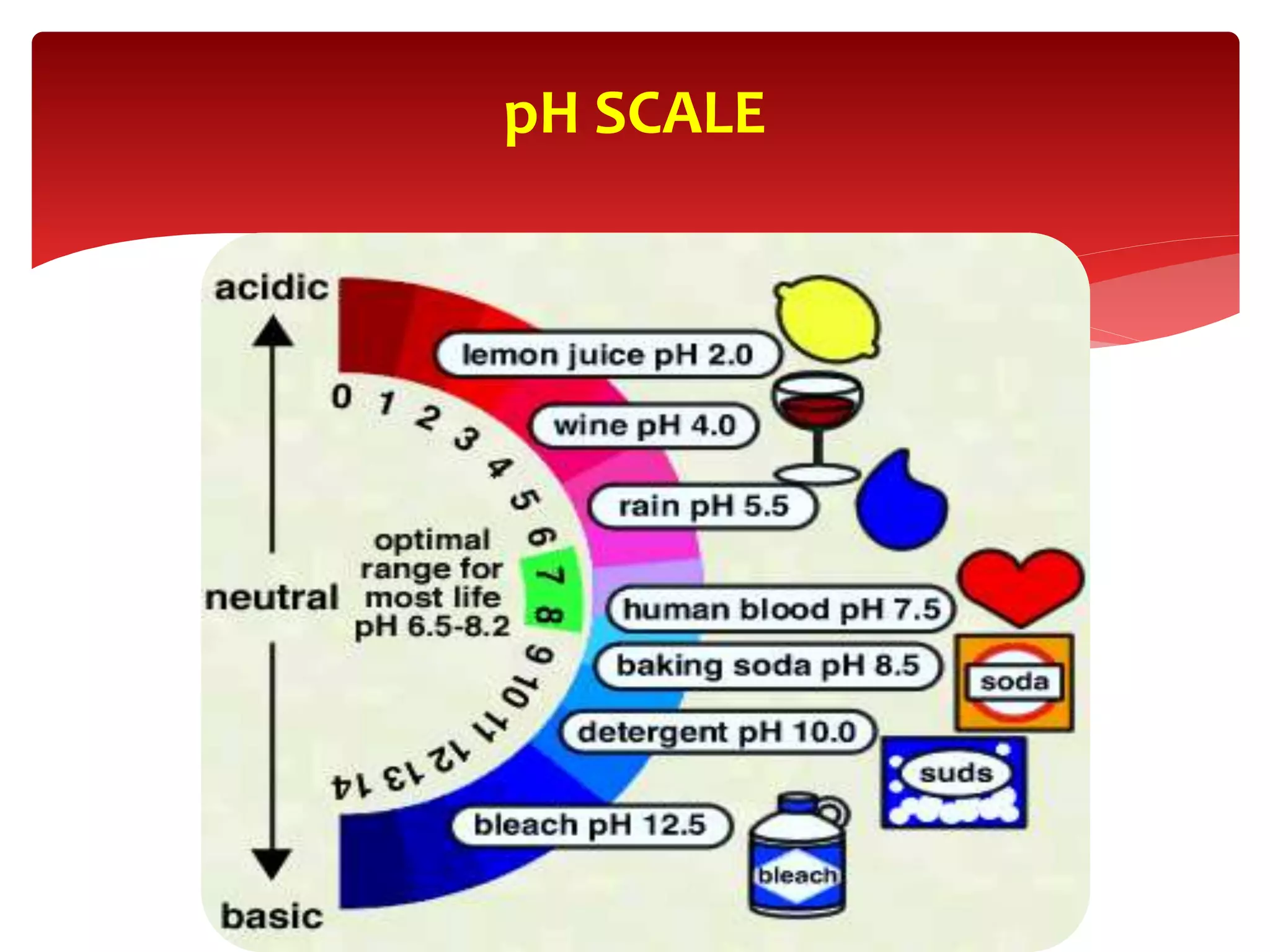
2) The physiology of the pH scale and how acids and bases affect hydrogen ion concentration.
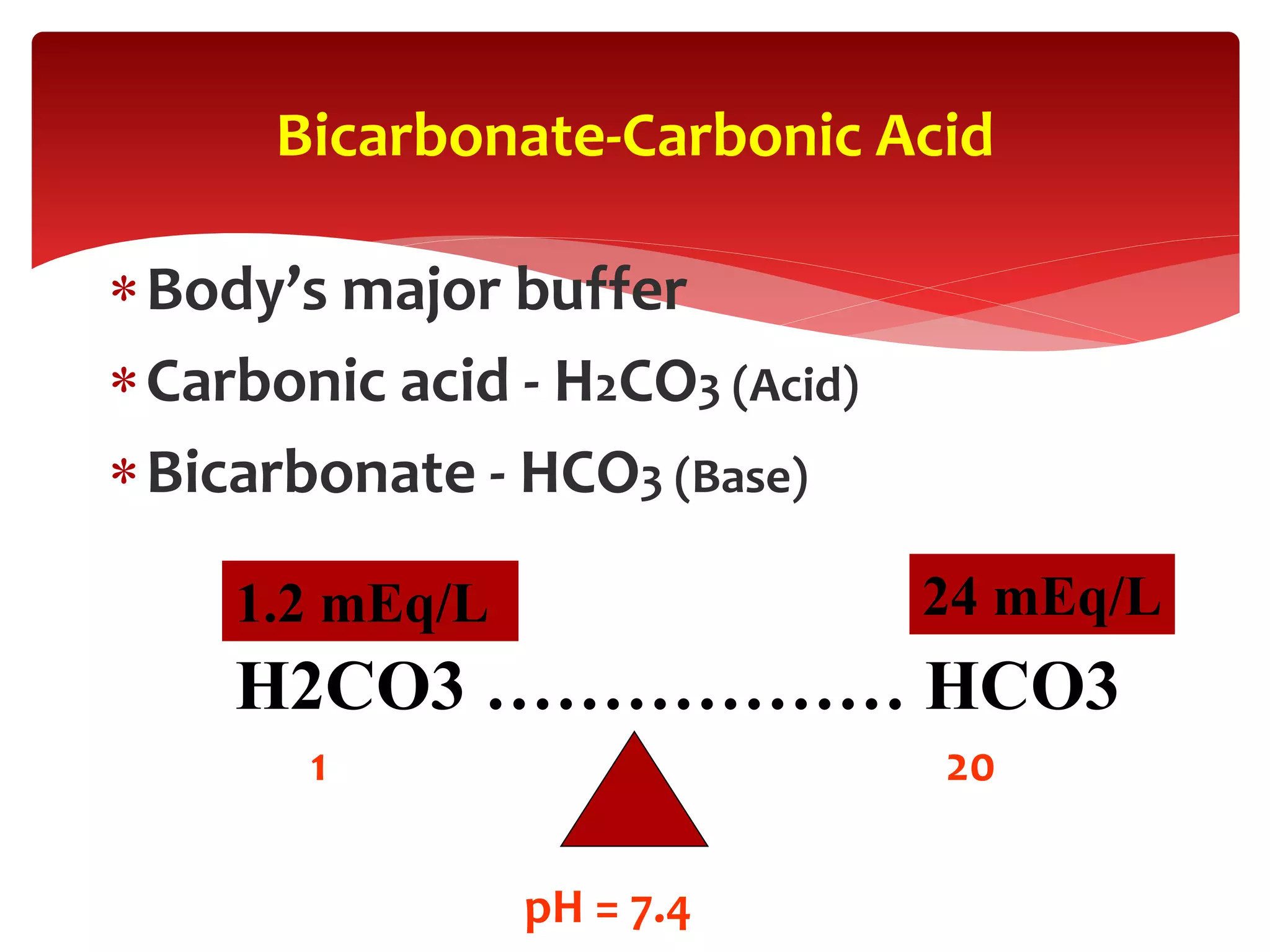
3) The key buffer systems that help maintain acid-base balance, including the important bicarbonate-carbonic acid buffer.
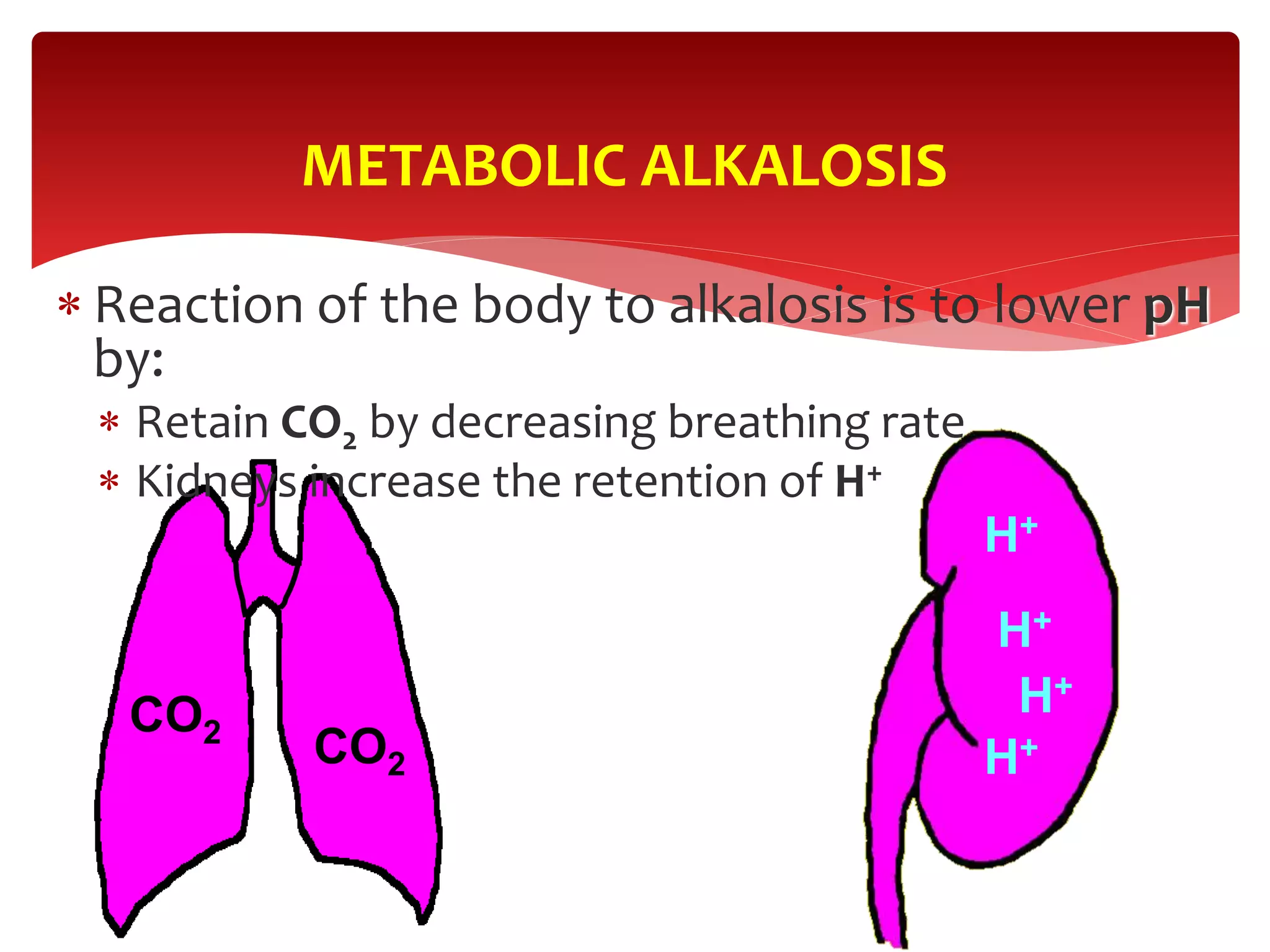
4) How the lungs and kidneys work to regulate acid-base balance through controlling carbon dioxide and bicarbonate levels respectively.
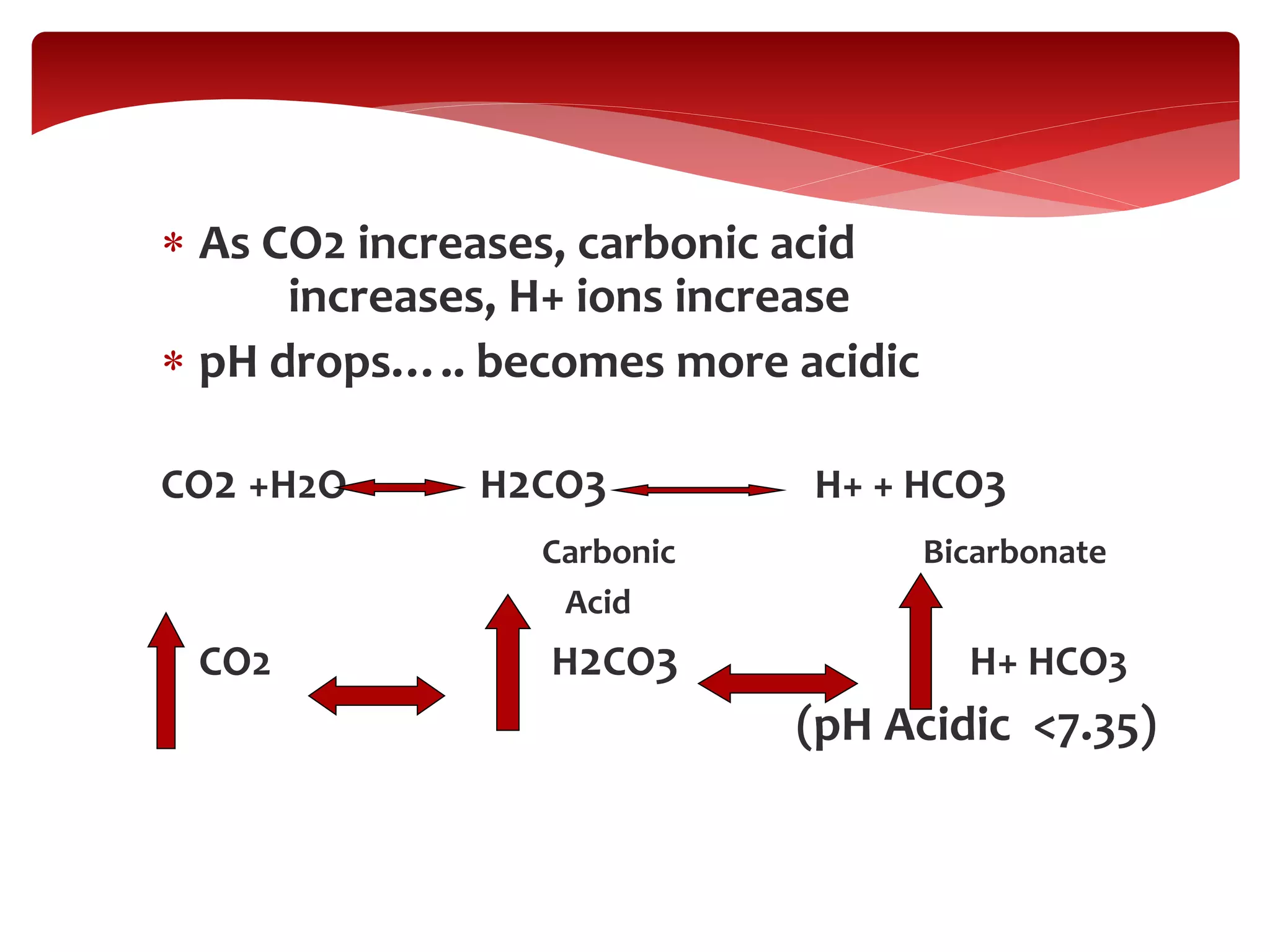
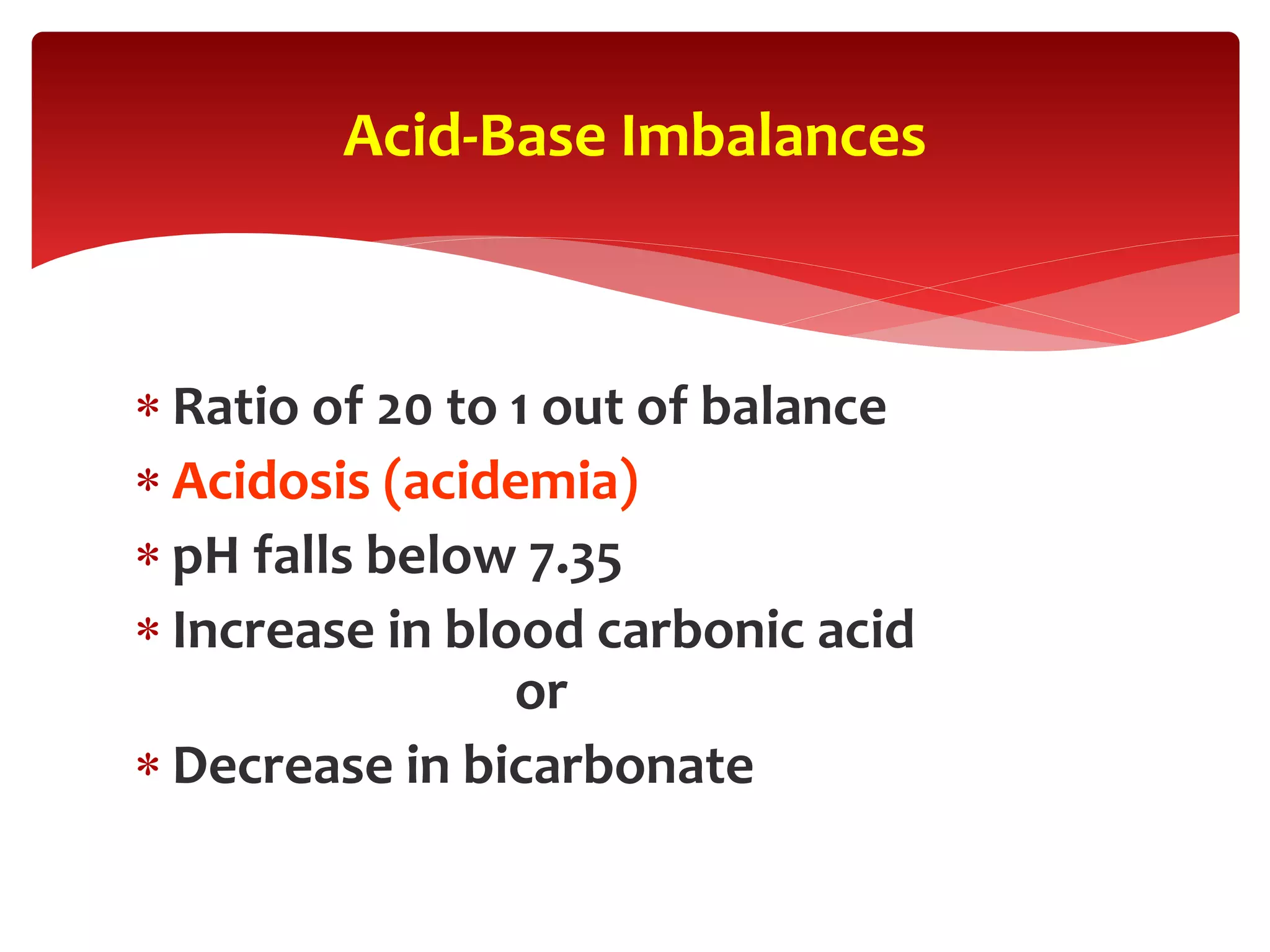
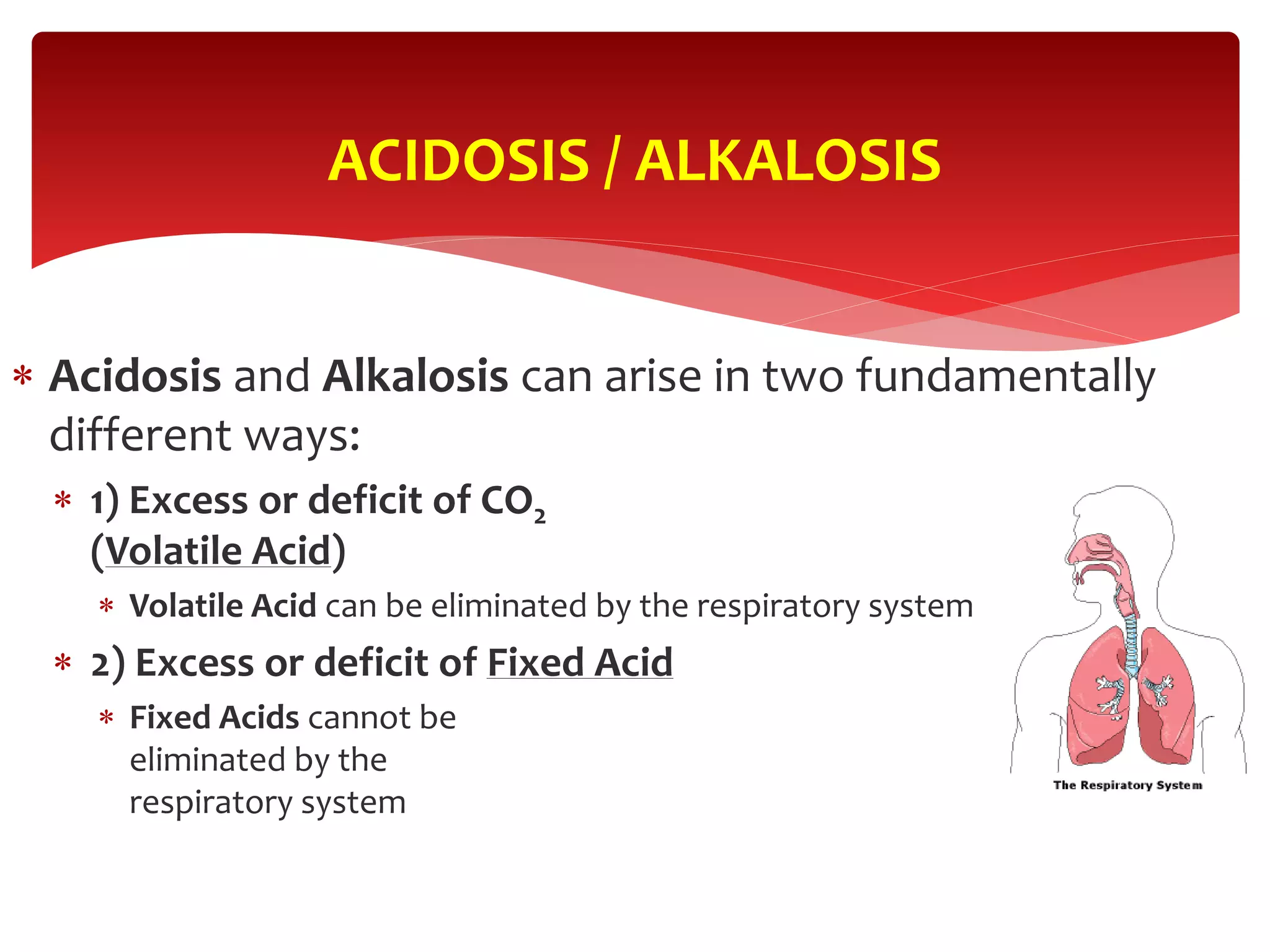
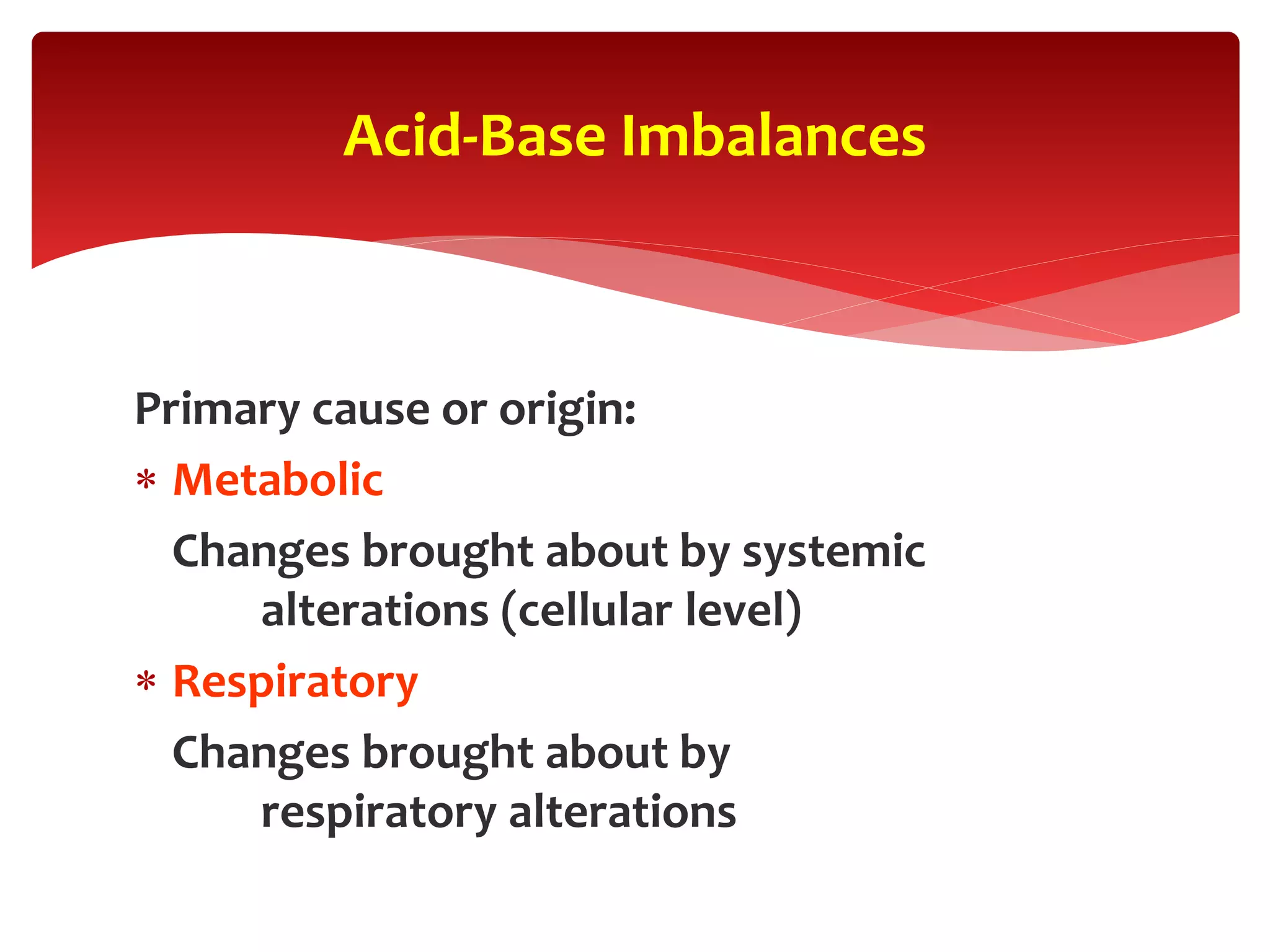
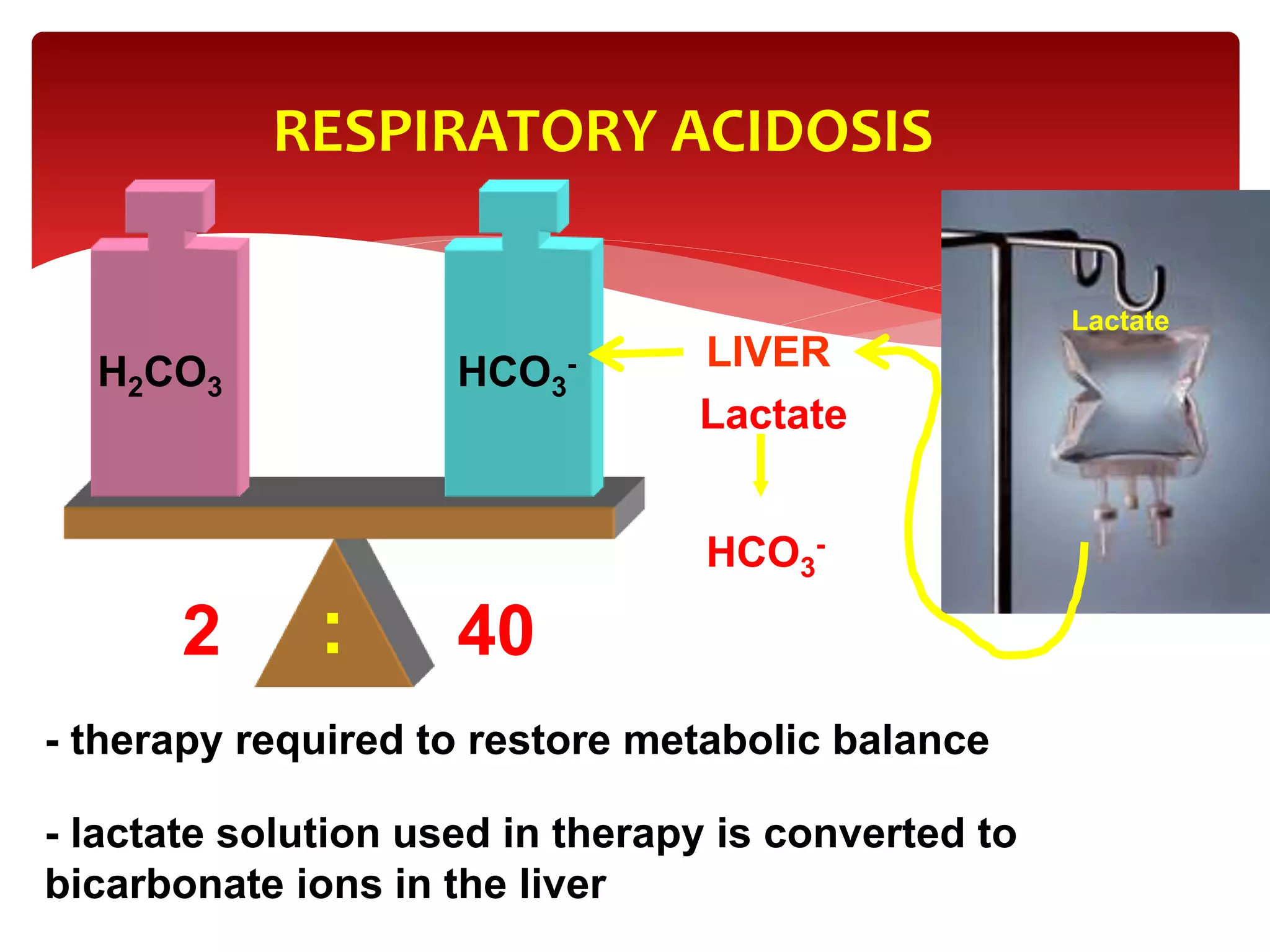
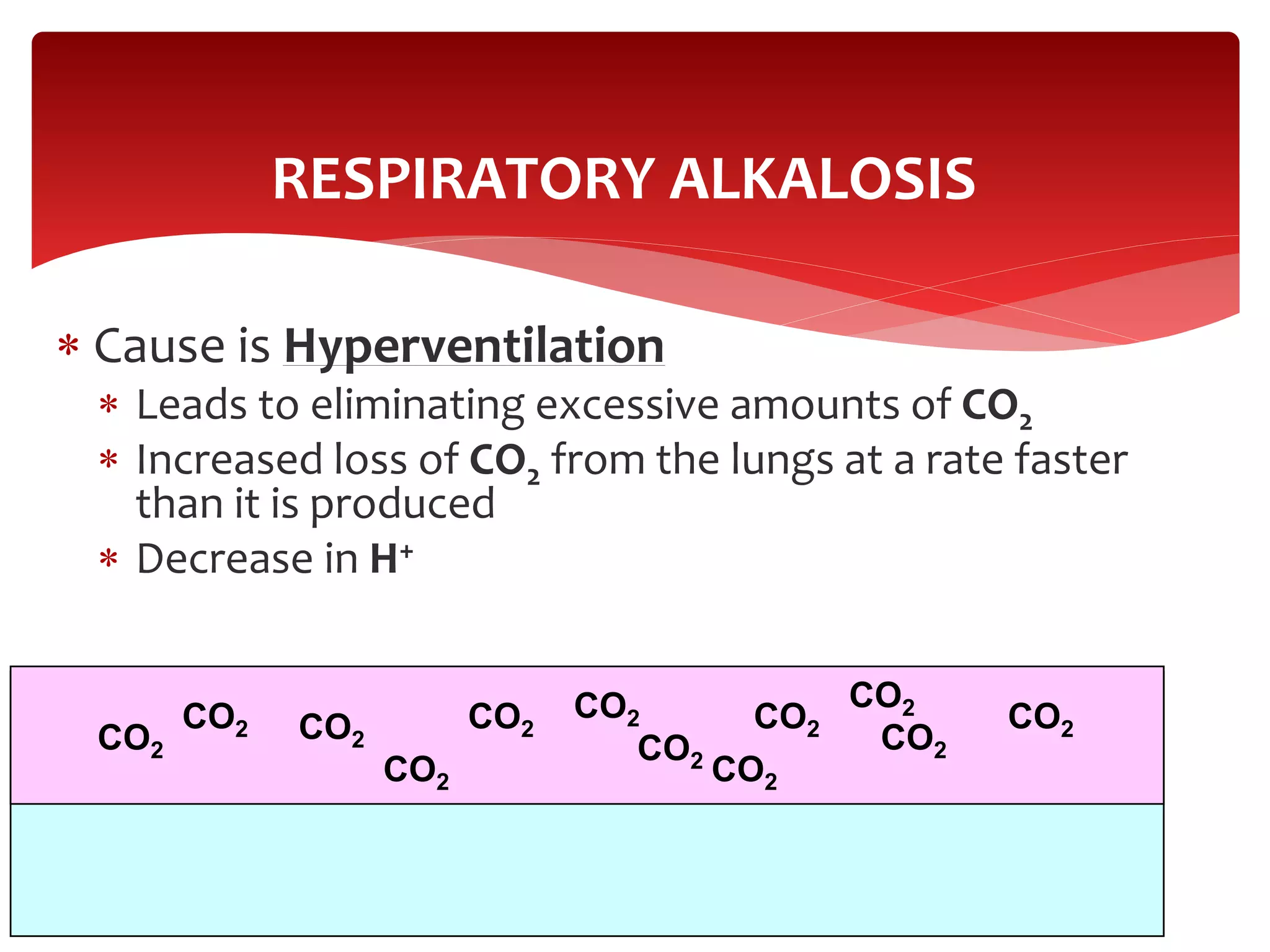
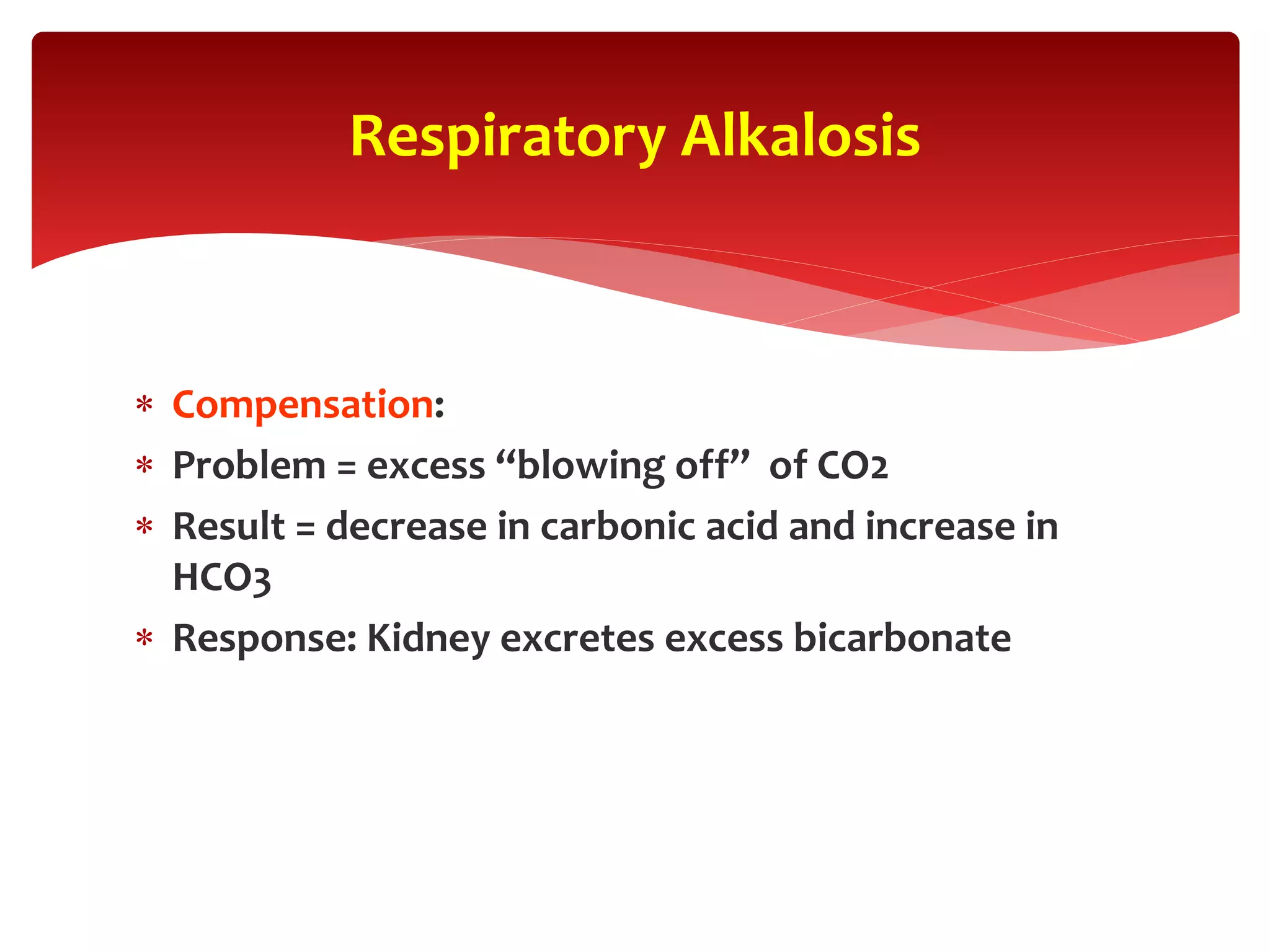
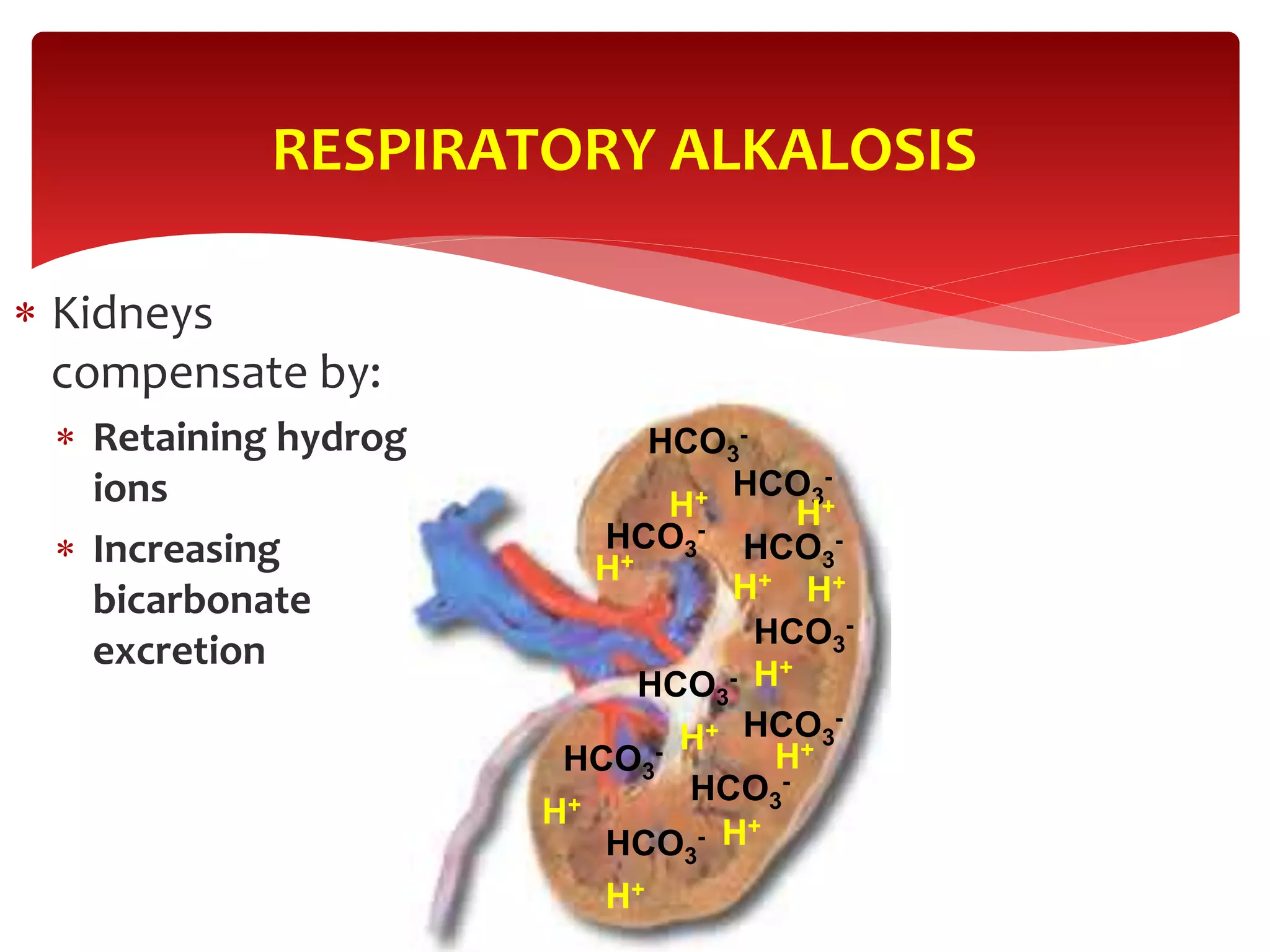
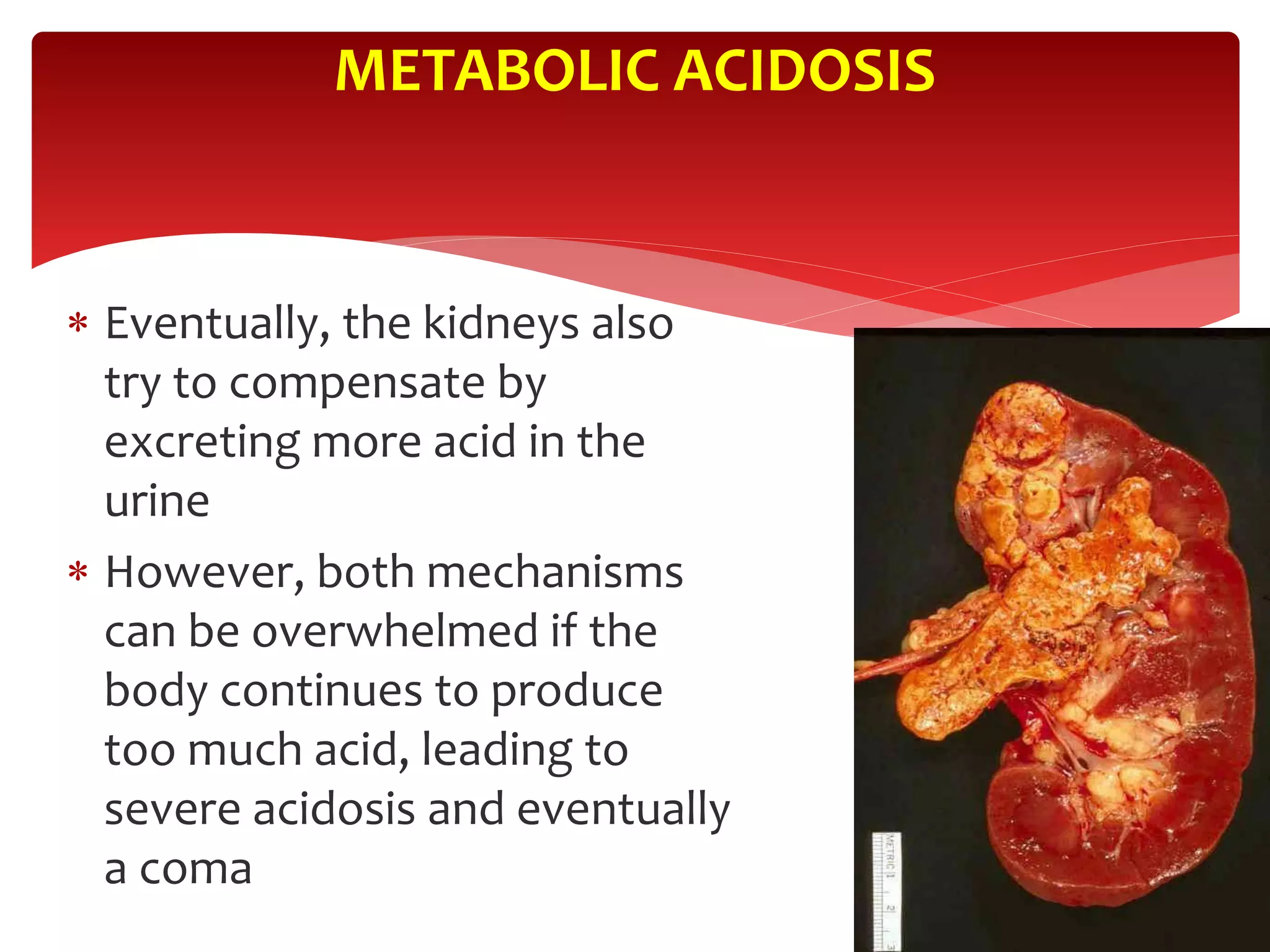
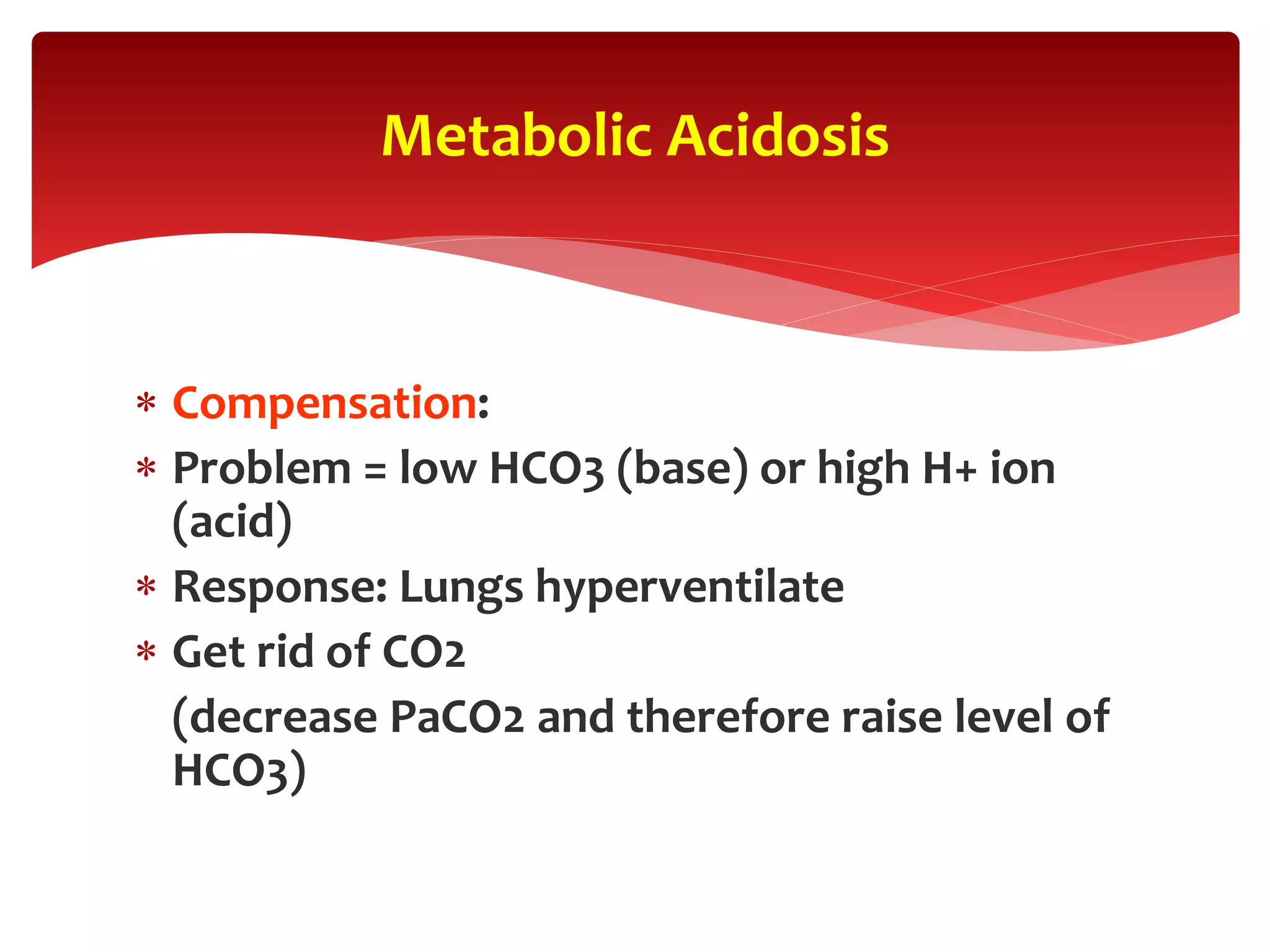
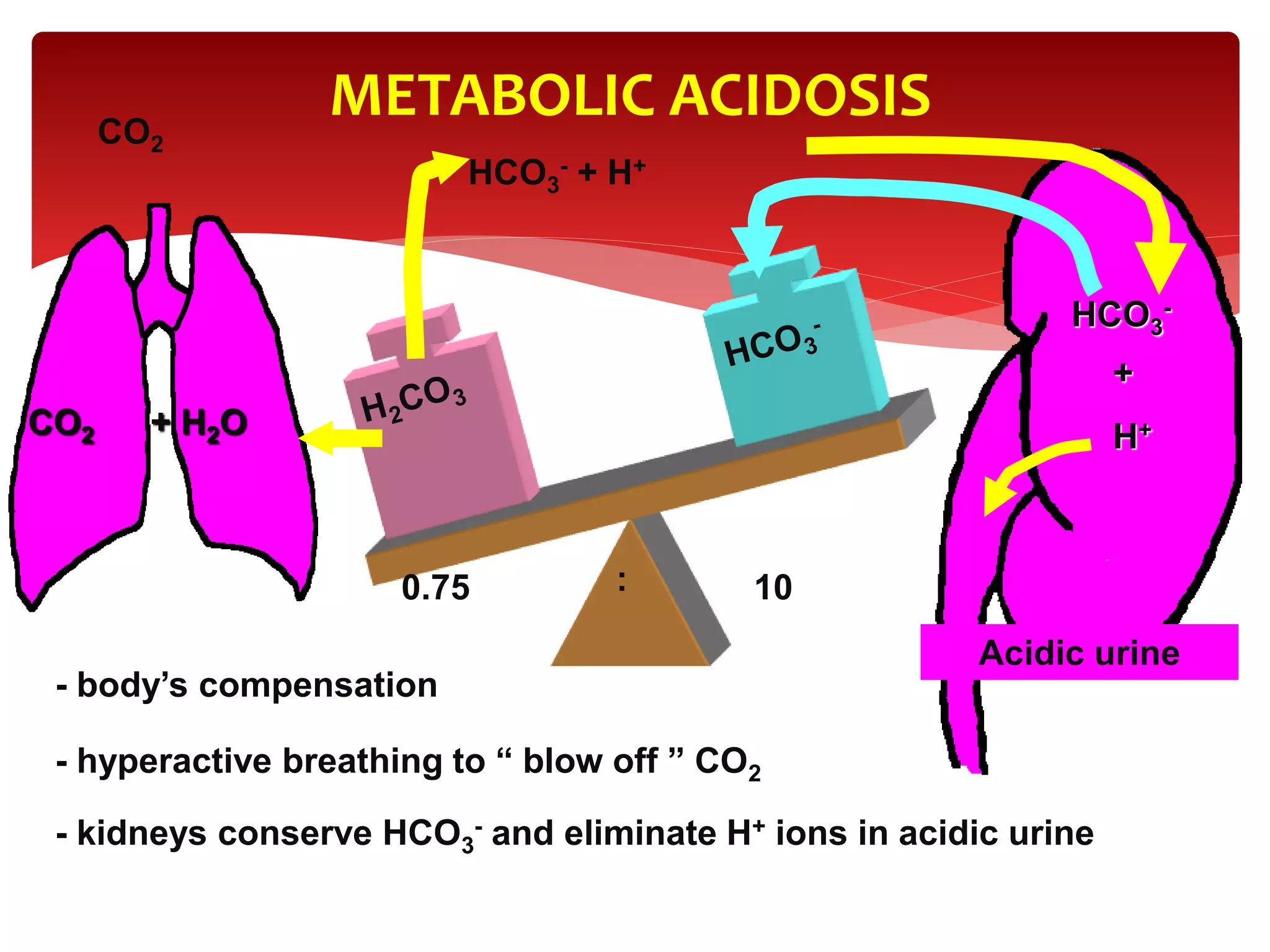
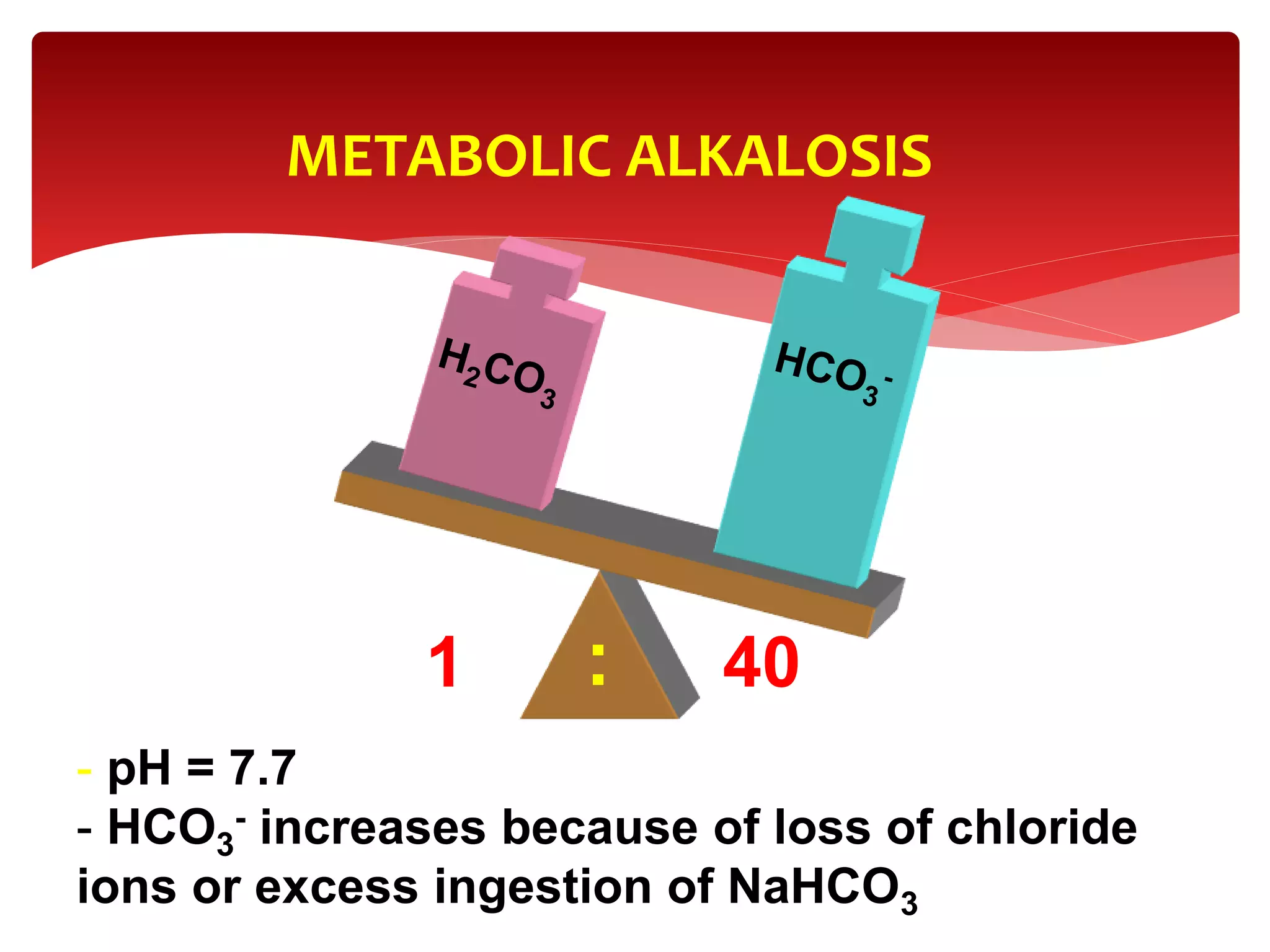
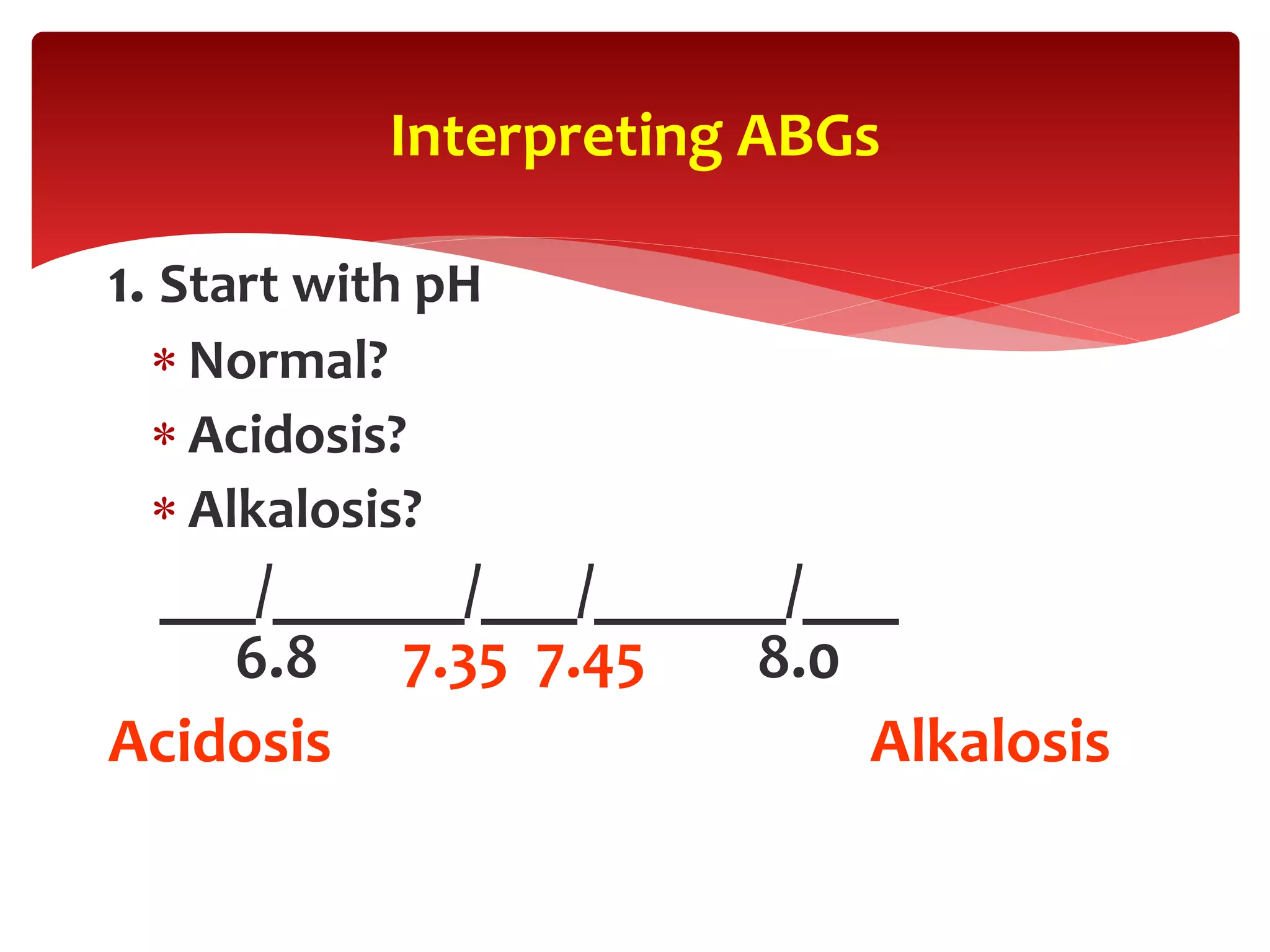
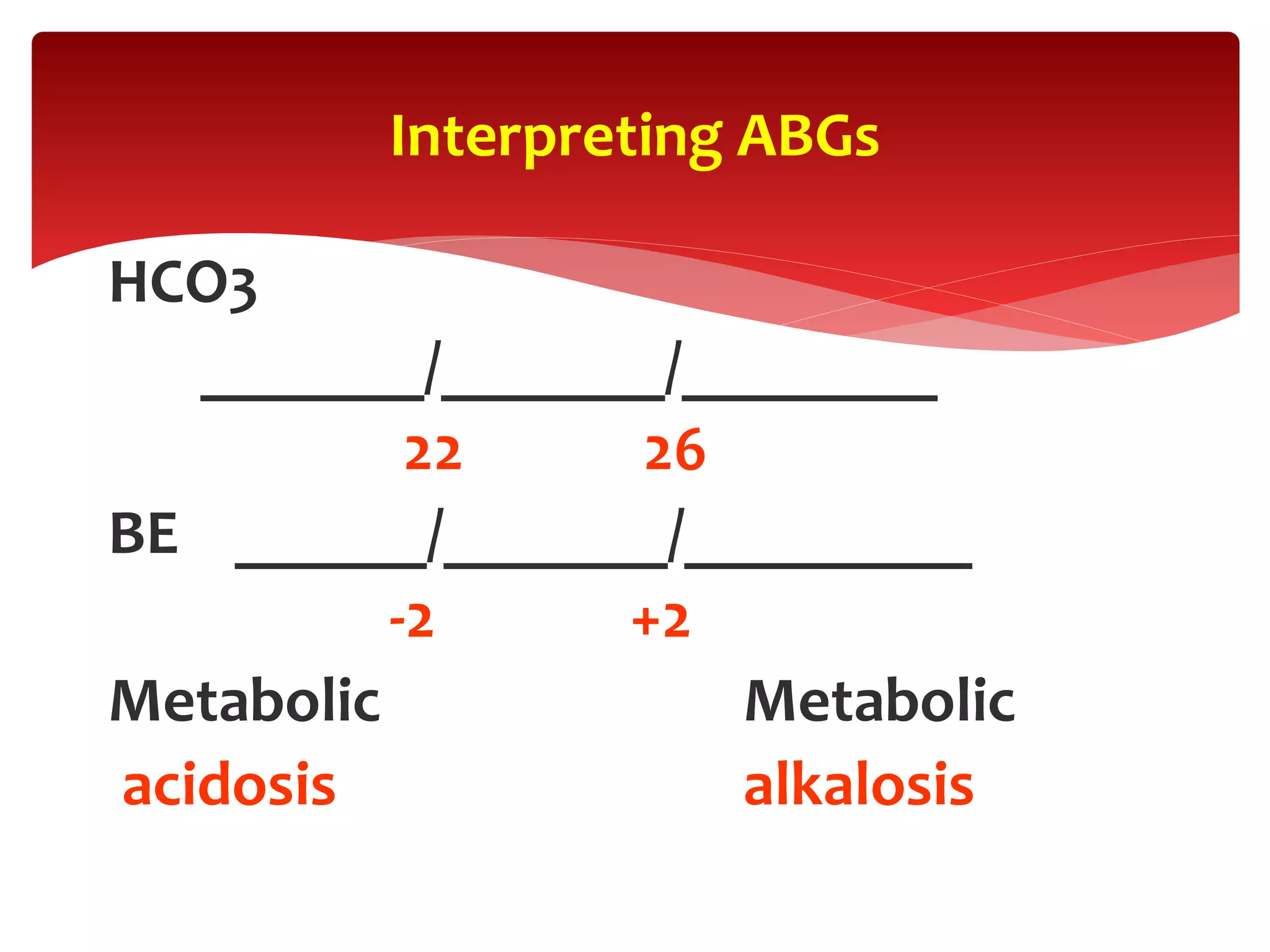
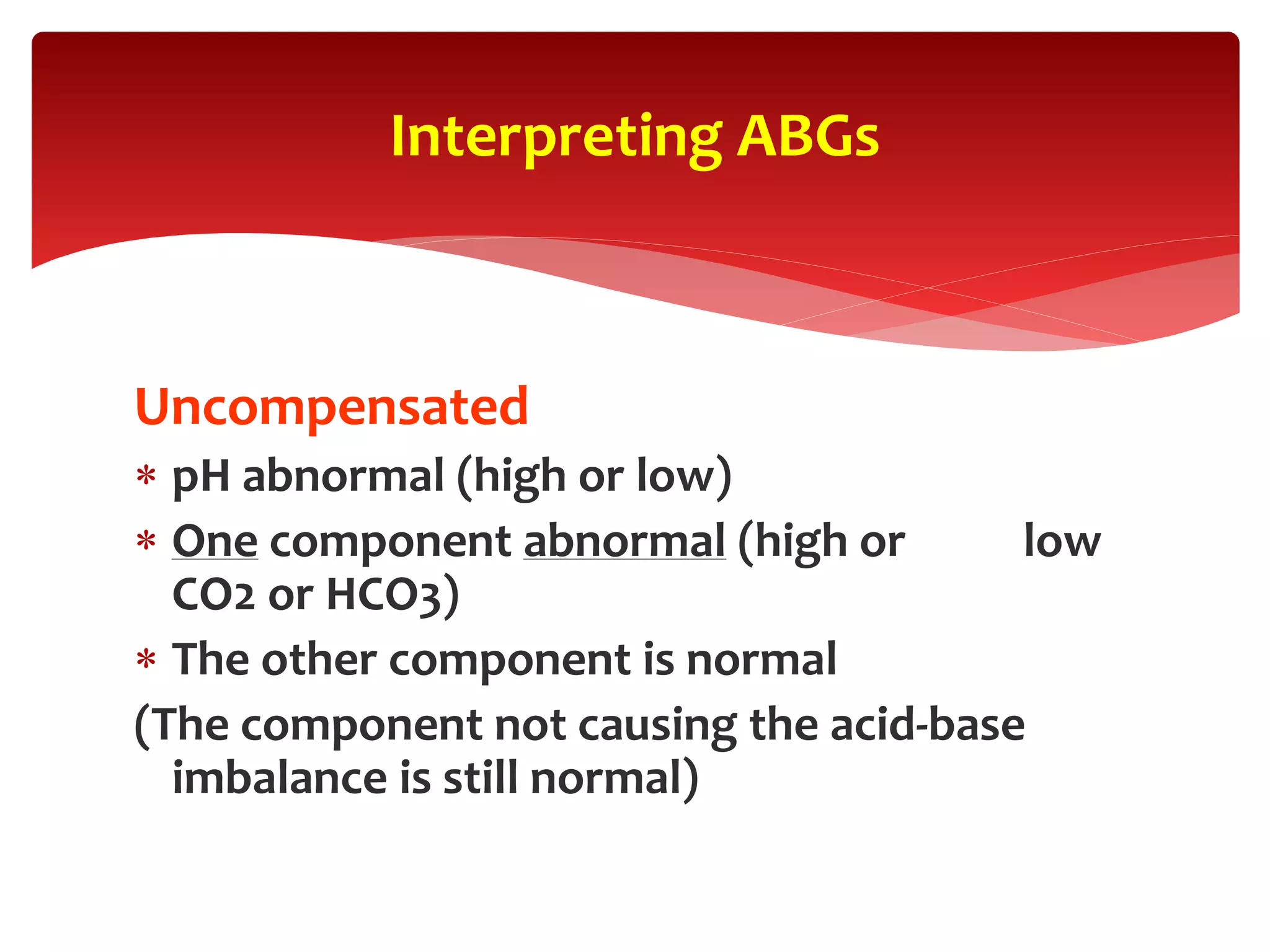
5) The four main types of acid-base imbalances: respiratory acidosis, respiratory alkalosis, metabolic


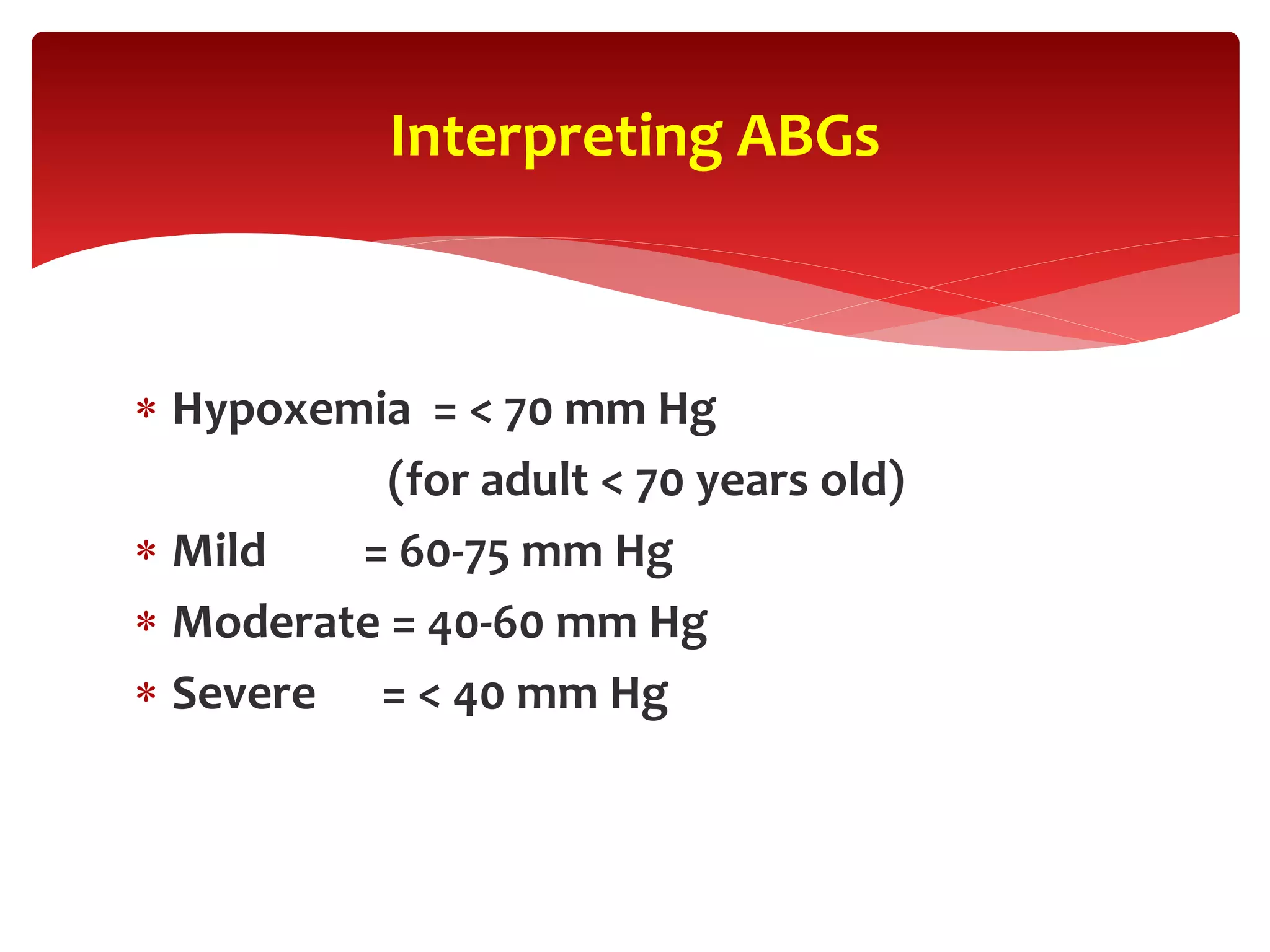
![ Oxygenation (PaO2). The PaO2 is the amount of oxygen
dissolved in the blood and therefore provides initial information
on the efficiency of oxygenation.
Ventilation (PaCO2). The adequacy of ventilation is inversely
proportional to the PaCO2 .so that, when ventilation increases,
PaCO2 decreases, and when ventilation decreases, PaCO2
increases.
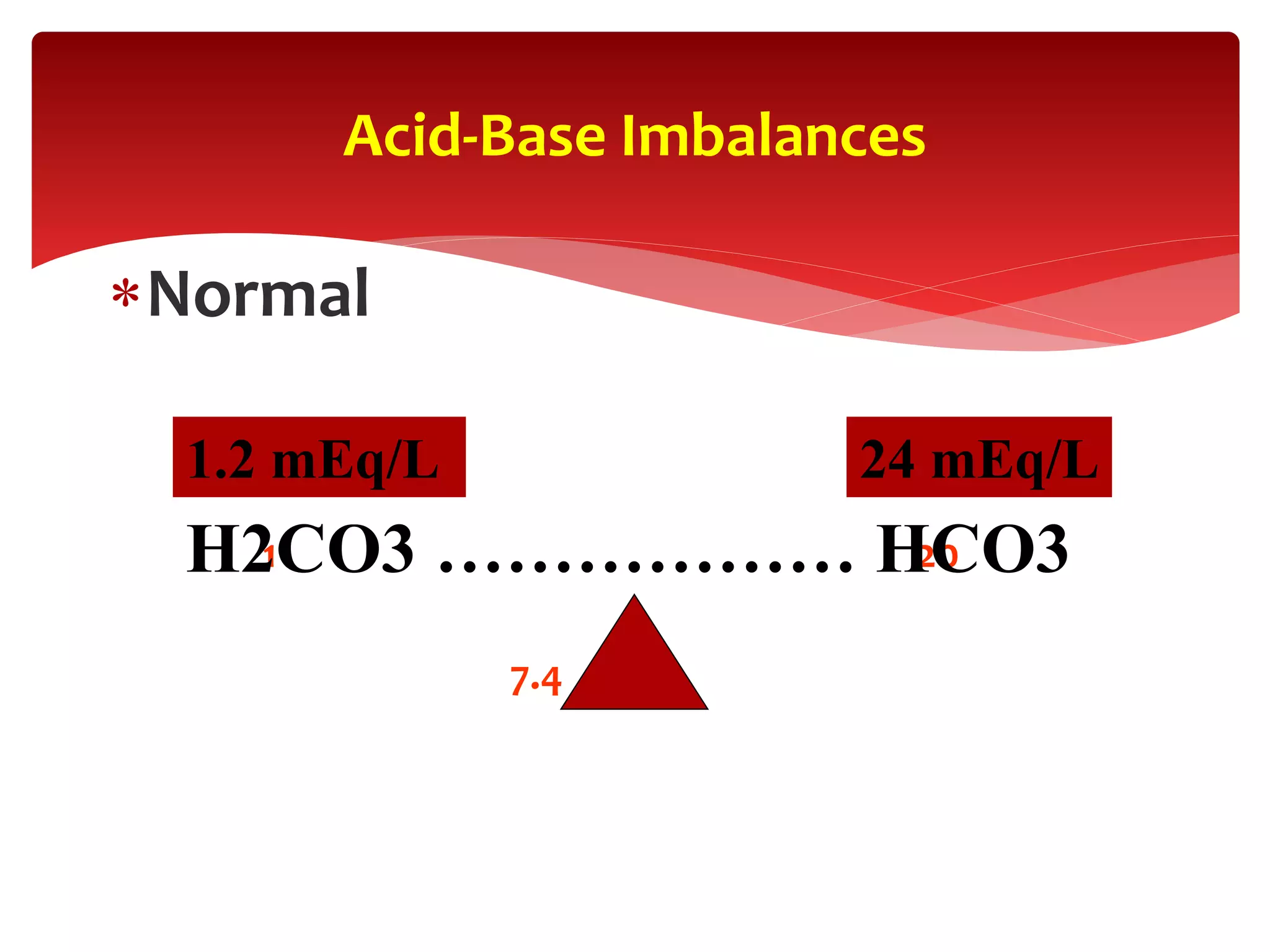
Acid-base status (pH, HCO3, and base deficit [BD]). A plasma pH
of >7.4 indicates alkalemia, and a pH of <7.35 indicates acidemia.
Despite a normal pH, an underlying acidosis or alkalosis may still
be present.
Arterial blood gas (ABG) provides an
assessment of the following:](https://image.slidesharecdn.com/acid-basebalance2-200720155824/75/Acid-base-balance-2-ppt-3-2048.jpg)



![ A strong acid is a substance that readily and almost
irreversibly gives up an H + and increases [H + ],
whereas a strong base avidly binds H + and decreases
[H + ].
Strong acids& base](https://image.slidesharecdn.com/acid-basebalance2-200720155824/75/Acid-base-balance-2-ppt-7-2048.jpg)
![ In contrast, weak acids reversibly donate H + ,
whereas weak bases reversibly bind H + ; both weak
acids and bases tend to have less of an effect on [H +
] (for a given concentration of the parent compound)
than do strong acids and bases.](https://image.slidesharecdn.com/acid-basebalance2-200720155824/75/Acid-base-balance-2-ppt-8-2048.jpg)


![ pH is the negative logarithm of the hydrogen
ion concentration ([H]). pH is a convenient
descriptor for power of hydrogen. Normally
the [H] in extacellular fluid is 40 nmol/L, a very
small number. By taking the negative log of
this value we obtain a pH of 7.4.
pH SCALE
pH = -log10(H+)](https://image.slidesharecdn.com/acid-basebalance2-200720155824/75/Acid-base-balance-2-ppt-11-2048.jpg)