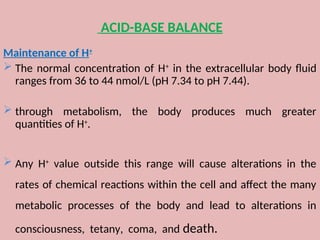
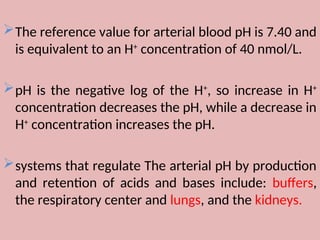
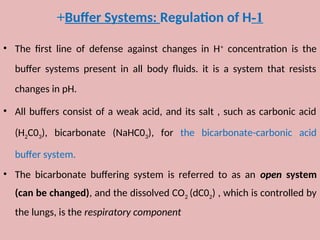
The document by Dr. Sanaa Nassar discusses acid-base balance, detailing the roles of hydrogen ions and buffers in maintaining pH levels in the body, with normal pH ranges identified. It explains various acid-base disturbances, including acidosis and alkalosis, their mechanisms, and the compensatory actions of the lungs and kidneys. The document also outlines specific causes for these disorders and how the body attempts to restore homeostasis through compensation.