The document discusses the significance of arterial blood gas (ABG) analysis in the management of critically ill patients in the ICU, emphasizing a systematic approach to diagnosing acid-base disorders. It details the normal ranges for pH, pCO2, and HCO3-, along with various conditions such as acute respiratory distress syndrome (ARDS), metabolic acidosis, and alkalosis, providing methods for calculating expected compensatory mechanisms. Additionally, it includes case studies that illustrate the clinical application of ABG interpretation and management strategies.




![ PaO2 and SaO2
PaO2 / FiO2 ratio
e.g. PaO2 :84 mmHg with 0.21 FiO2 [room air]
PaO2 / FiO2 = 84 / 0.21 = 400
Normal = 300 – 500 mmHg
< 300 = acute lung injury [previous definition]
< 200 = ARDS [previous definition]
Berlin definition:
200 – 300 [with PEEP/CPAP > 5] = mild ARDS
< 200 [with PEEP > 5]
= moderate ARDS
<100 [with PEEP > 5]
= severe ARDS](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-5-320.jpg)

![ The pH is the –log [H]. So by altering either the PCO2
or the HCO3-, [H] will change, and so will pH.
An acidemia(low pH) can result from either a low
HCO3- or a high CO2
An alkalemia (high pH) can result from either a high
HCO3 or a low CO2](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-7-320.jpg)



![ Normal pH is 7.4
Calculate the change in pH (from 7.4)
A.
in acute respiratory disorder (acidosis / alkalosis)
change in pH = 0.008 X [PaCO2 -40]
expected pH = 7.4 +/-change in pH
B. in chronic respiratory disorder (acidosis/alkalosis)
change in pH = 0.003 X [PaCO2 -40 ]
expected pH = 7.4 +/- change in pH
Compare the pH on ABG
if pH on ABG is close to A, it is acute disorder
if pH on ABG is close to B, it is chronic disorder](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-11-320.jpg)
![ M/60 yrs, k/c/o C.O.P.D. admitted with U.T.I.
ABG: 7.26 / 84 / 74 / 37 / 94%
[A]. For Acute change in pH;
change in pH = 0.008 X [84 – 40 ] =0.008 X [44] =0.35
Expected pH = 7.4 – 0.35 = 7.05
[B]. For chronic change in pH’
Change in pH = 0.003 X [84 – 40 ] = 0.003 X [44] = 0.13
Expected pH =7.4 -0.13 = 7.27
So B is near to the patient’s ABG which is 7.26; so primary disorder
is chronic respiratory acidosis.](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-12-320.jpg)
![Primary
disorder
Initial Compens Compensa
chemic a-tory
al
response tory
change
mechanis
s
m
Respiratory
acidosis
Expected level of compensation
↑PCO2 ↑HCO3-
Acute
Bufferingrule of 1
↑[HCO3-] = 1 mEq/L for every
10 mmHg delta PCO2
Chronic
Generation
of new
HCO3 –
rule of 3
[HCO-] = 3 mEq/L for every
10mmHg delta PCO2](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-13-320.jpg)
![Primary
disorder
Initial
chemic
al
change
s
Compen
sa-tory
respons
e
Compensatory
mechanism
Expected level of
compensation
Respiratory
alkalosis
↓PCO2 ↓HCO3-
Acute
buffering- rule
of 2
↓[HCO-] = 2 mEq/L for
every 10 mmHg delta PCO2
chronic
Decreased
reabsorption
of HCO3- rule
or 4
↓[HCO3-] = 4 mEq/L for every
10 mmHg delta PCO2](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-14-320.jpg)
![ Metabolic acidosis results from a primary decrease in
plasma [HCO3-]
Check the respiratory compensation by winter’s
formula:](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-15-320.jpg)
![Primary
disorder
Initial
chemica
l
changes
Compens Compensaa-tory
tory
response mechanism
Expected level of
compensation
Metabolic
acidosis
↓HCO3-
↓PCO2
Hyperventilation
Metabolic
alkalosis
↑HCO3-
↑PCO2
Hypoventilation PCO2 = 0.7 X [HCO3] +
21+/- 2
PCO2 = 1.5 X [HCO3-]+
8 +/-2](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-16-320.jpg)
![ 7.23 / 34 /88 /17 [ metabolic acidosis]
Winter ‘s formula:
Expected PaCO2 = 1.5 X [HCO3-] + 8 +/- 2
= 1.5 X [17] + 8 +/ -2
= 33.5 + / -2 = 31.5 – 35.5 mmHg
7.45 / 43 / 95 / 30 [ metabolic alkalosis]
Winter ‘s formula:
Expected PaCO2= 0.7 X [HCO3-] + 21 + / - 2
= 0.7 X [30] + 21 + / - 2
=42 + / -2 = 40 – 44 mmHg](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-17-320.jpg)
![ Metabolic alkalosis reflects an increase in plasma
[HCO3-]
It can be classified into saline responsive or
nonresponsive.
More than 20 mEq/L urinary chloride is saline
unresponsive and less than 20 mEq/L is saline
responsive.](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-18-320.jpg)


![ It is used to determine if a metabolic acidosis is due to
an accumulation of non- volatile acids [e.g. lactic
acidosis] or a net loss of bicarbonate [e.g. diarrhea]
Na + UC = [ Cl + HCO3 ] + UA
UA – UC [ Anion gap] = Na –[ Cl + HCO3- ]
AG= Na –[Cl + HCO3]; normal AG is 12+/-2 mEq/L](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-21-320.jpg)
![Unmeasured Anions
Unmeasured Cation
Albumin: 15 mEq/L
Calcium: 5 mEq/L
Organic Acids: 5 mEq/L
Potassium: 4.5 mEq/L
Phosphate: 2 mEq/L
Magnesium: 1.5 mEq/L
Sulfate: 1 mEq/L
Total UA: 23 mEq/L
Total UC: 11 mEq/L
Anion AG = UA – UC = 12 mEq/L
Adjusted AG = calculated AG + 2.5 X [4 – S.albumin gm%]](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-22-320.jpg)
![ 7.23 / 34 /88 /17 : Metabolic Acidosis
Na : 135 / Cl: 99 / K: 3.5
AG = Na - [ Cl + HCO3-] = 135 – [ 99 + 17] = 19
High AG](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-23-320.jpg)
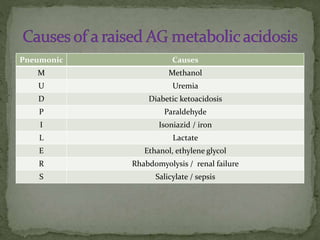

![ Check urinary AG in non-AG metabolic acidosis
U Na + U K – U Cl
Normal :
negative
Non-renal loss of bicarbonate [diarrhea] : negative
Renal loss of bicarbonate[ RTA /
H+ excretion]
: positive](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-26-320.jpg)
![ In less obvious cases, the coexistence of two metabolic
acid-base disorders may be apparent by calculating the
difference between the change in AG [delta AG] and
the change in serum HCO3- [delta HCO3-].
e.g. Diabetic ketoacidosis
This is called the Delta gap or gap –gap.](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-27-320.jpg)

![ 7.23 / 34 /88 /17 : Metabolic Acidosis
Na : 138 / Cl: 99 / K: 3.5
AG = Na - [ Cl + HCO3-] = 138 – [ 99 + 17] = 22
Next step is to calculate the Delta Gap.
Delta AG = patient’s AG -12 = 22 – 12 = 10
Delta HCO3- = 24 – patient’s HCO3- = 24 – 17 = 7
Delta gap = Delta AG- Delta HCO3- = 10 – 7 = 3
Additional metabolic alkalosis is also present with high AG
metabolic acidosis.](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-29-320.jpg)




![ ABG: 7.23/17/235/7 with 50% FiO2 on V.M.
Winter’s formula:
Expected PaCO2 = 1.5 X [7] + 8 +/- 2 = 18.5 +/-2](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-34-320.jpg)
![ ABG: 7.23/17/235/7 with 50% FiO2 on V.M.
Electrolytes: Na: 123 mEq/L, Cl: 97 mEq/L, S.K 3.5
AG = Na – [Cl +HCO3-] = 123 – [97 + 7] = 19
High AG metabolic acidosis
Delta gap = Delta AG – Delta HCO3-
= [19 - 12 ] – [24 – 7 ]
=7–7=0
Non –anion gap metabolic acidosis
](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-35-320.jpg)





![ For Acute disorder;
Change in pH = 0.008 X [65 – 40 ]=0.2
Expected pH = 7.40 – 0.2 = 7.20
For chronic disorder;
Change n pH= 0.003 X [65 – 40 ] = 0.07
Expected pH = 7.40 – 0.07 = 7.33
So its chronic respiratory acidosis.](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-41-320.jpg)






![ Winter’s formula
Expected PaCO2= 1.5 X [16] + 8 +/ -2
= 32 -/+ 2 = 30 – 34 mEq/L
So it is fully compensated metabolic disorder.](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-48-320.jpg)
![ AG = Na – [Cl + HCO3-] = 134 – [104 + 16 ] = 14](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-49-320.jpg)





![ Expected PaCO2 = 0.7 X [ HCO3-] + 21 +/- 2
= 0.7 X [ 45 ] + 21 +/ - 2
= 52.5 +/-2
= 50.5 – 54.5](https://image.slidesharecdn.com/abganalysis-140302091527-phpapp01/85/Abg-analysis-55-320.jpg)




