This document provides an overview of the pathophysiology of pH and acid-base homeostasis. It discusses:
1. The definition of pH and normal pH levels in the body. Acids donate hydrogen ions while bases accept them.
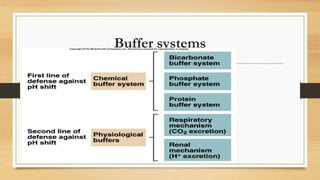
2. The two main buffer systems that help regulate pH - the bicarbonate buffer system and protein buffers. The Henderson-Hasselbalch equation describes the relationship between bicarbonate, carbonic acid and pH.
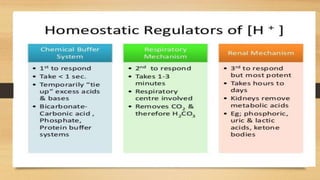
3. The mechanisms that generate and regulate acids and bases in the body, including the roles of respiration, the kidneys, and various buffer systems.
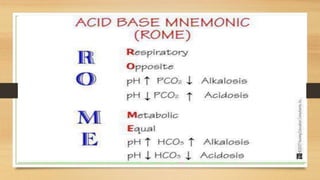
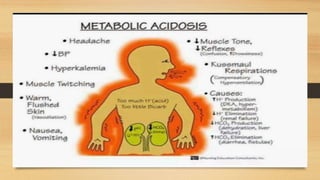
4. The classifications of acid-base disturbances including respiratory and metabolic acidosis/alk

![INTRO.
• pH is the negative logarithm of [H+] in a solution.
• pH of blood is 7.4 (+0.05)
• Normal pH of body fluids:
Arterial blood is 7.4
Venous and interstitial pH is 7.35
intracellular pH is 7.0](https://image.slidesharecdn.com/pathophysiologyofph-180914073055/85/Pathophysiology-of-ph-2-320.jpg)





![Handerson-Hasselbalch equation
• The relationship between the conc. Of a weak acid and its conjugate base in
solution is well illustrated by the this equation.
• pH=pKa(H2CO3)+Log10 [HCO3
-]
[ H2CO3]
Since the conc. Of H2CO3 is proportional to PCO2, and the pKa of H2CO3 is 6.1,
the equation can be re-written as
pH=6.1+Log10 [HCO3
-]
[0.0307xPCO2]](https://image.slidesharecdn.com/pathophysiologyofph-180914073055/85/Pathophysiology-of-ph-13-320.jpg)
![Henderson-Hasselbalch Equation cont.
• Key.
o Pka=6.1
o α = 0.03
o PaCO2= 40mmHg
o [HCO3
-] = 24mmol/L
o pH=7.4
By keying in the variables, the ratio of
HCO3
-:H2CO3= 20:1
• pH=6.1+ log 24
0.03x40
pH= 6.1 + log 20
1
pH=7.4](https://image.slidesharecdn.com/pathophysiologyofph-180914073055/85/Pathophysiology-of-ph-14-320.jpg)




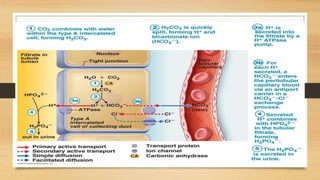










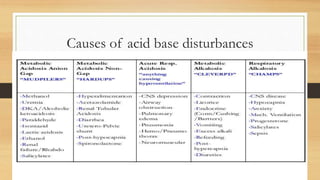


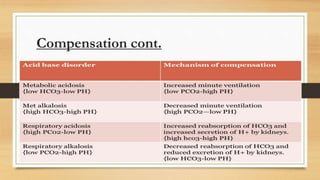
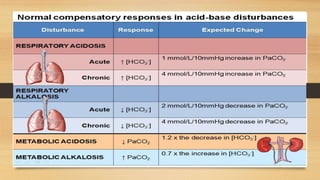
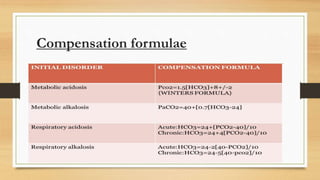
![Anion gap concept (?Delta concept)
• Defined as the difference between measured cations and measured anions.
Serum AG= [Na]-[Cl+HCO3]
NORMAL=12+3 (varies depending on laboratory).....What if the anion gap is outside these figures?
• It is a concept of metabolic acidosis and it helps us to know if metabolic acidosis is due
to ;
Loss of bicarbonate
Accumulation of non volatile acids ( organic anions)
• Provides an index of the relative concentration of plasma anions other than chloride and bicarbonate.
• Based on this concept, met. Acidosis is categorized into anion gap [normochloremic]and non-anion
gap[normal anion gap/hyperchloremic] metabolic acidosis.](https://image.slidesharecdn.com/pathophysiologyofph-180914073055/85/Pathophysiology-of-ph-41-320.jpg)
![Anion gap cont.
• NB: The law of electroneutrality dictates that the number of cations and anions in
any open system hence anion ‘gap’ should not exist.
• However, since there are many anions and cations that are not routinely measured in
the lab. There exists an anion gap when calculated[using the measured ions]
• Unmeasured anions are much more than unmeasured cations hence the anion gap.
• Unmeasured anions include: albumin, phosphates, sulfates, ketones and lactate](https://image.slidesharecdn.com/pathophysiologyofph-180914073055/85/Pathophysiology-of-ph-42-320.jpg)

![Causes of high anion gap (normochloremia)
• Most commonly caused by met. Acidosis in which negatively charged acids e.g
ketones[DKA, alcohol, starvation], lactate[lactic acidosis],sulfates or metabolites of
methanol, ethylene glycol or salicylate are buffered by HCO3.
• Other causes of increased anion gap include:
a) Hyperalbuminemia increased anions
b) Uremia
c) Hypocalcemia
d) Hypomagnesemia decreased cations](https://image.slidesharecdn.com/pathophysiologyofph-180914073055/85/Pathophysiology-of-ph-44-320.jpg)


![Delta gap
• Is the difference between patient’s anion gap and the normal(reference)anion gap.
• This amount is considered an HCO3
- equivalent since for every unit rise in the anion
gap, the [HCO3
- ]should lower by 1(by buffering)
• If the delta gap is added to the measured HCO3
- the result should be in the normal
range for HCO3
-; elevation indicates the additional presence of met. Alkalosis.
• If metabolic acidosis is present, a delta gap is calculated to identify concomitant
metabolic alkalosis.](https://image.slidesharecdn.com/pathophysiologyofph-180914073055/85/Pathophysiology-of-ph-47-320.jpg)

![Base Excess/Base deficict
• Amount of strong base/acid needed to be added/subtracted from a substance in order to return
the pH to normal. [change in conc. of buffer base]
• Refer to an excess or deficit, respectively, in the amount of base present in the blood.
• By measuring blood pH against ambient PaCO2 and against a PaCO2 of 40mmhg.
• BE is associated with abnormality in HCO3 so it is influenced by a metabolic process.
• BB={HCO3 -24mEq/L}+{proteins-15mEq/L}+{Hb/HbO2-9mEq/L}=48 mEq/L.
• A value outside of normal range[-2 to +2mEq/l] suggests a metabolic cause for the disorder.
• A base excess>+2mEq/l indicates a metabolic alkalosis.
• A base excess of -2mEq/l indicates a metabolic acidosis.](https://image.slidesharecdn.com/pathophysiologyofph-180914073055/85/Pathophysiology-of-ph-50-320.jpg)