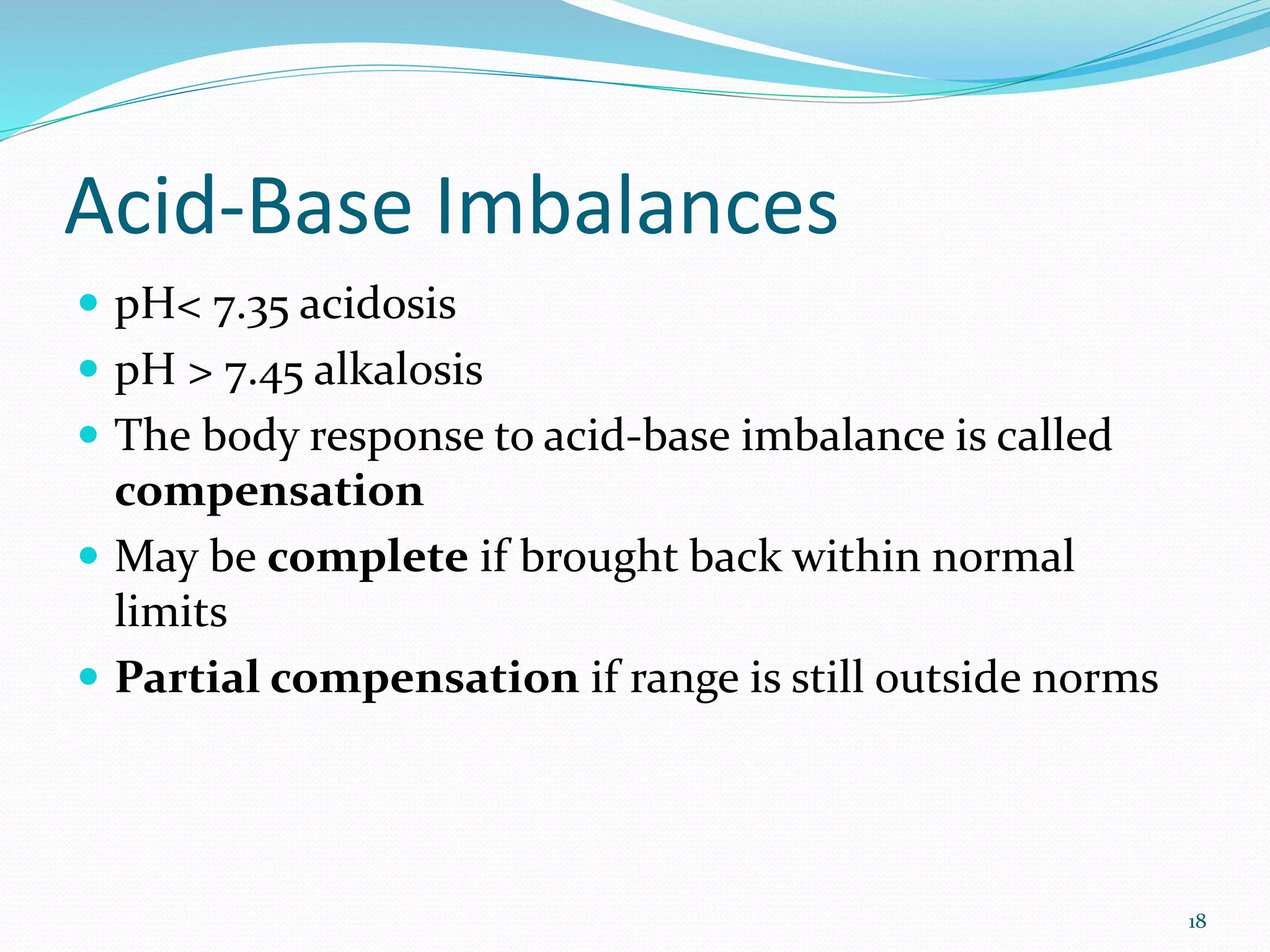
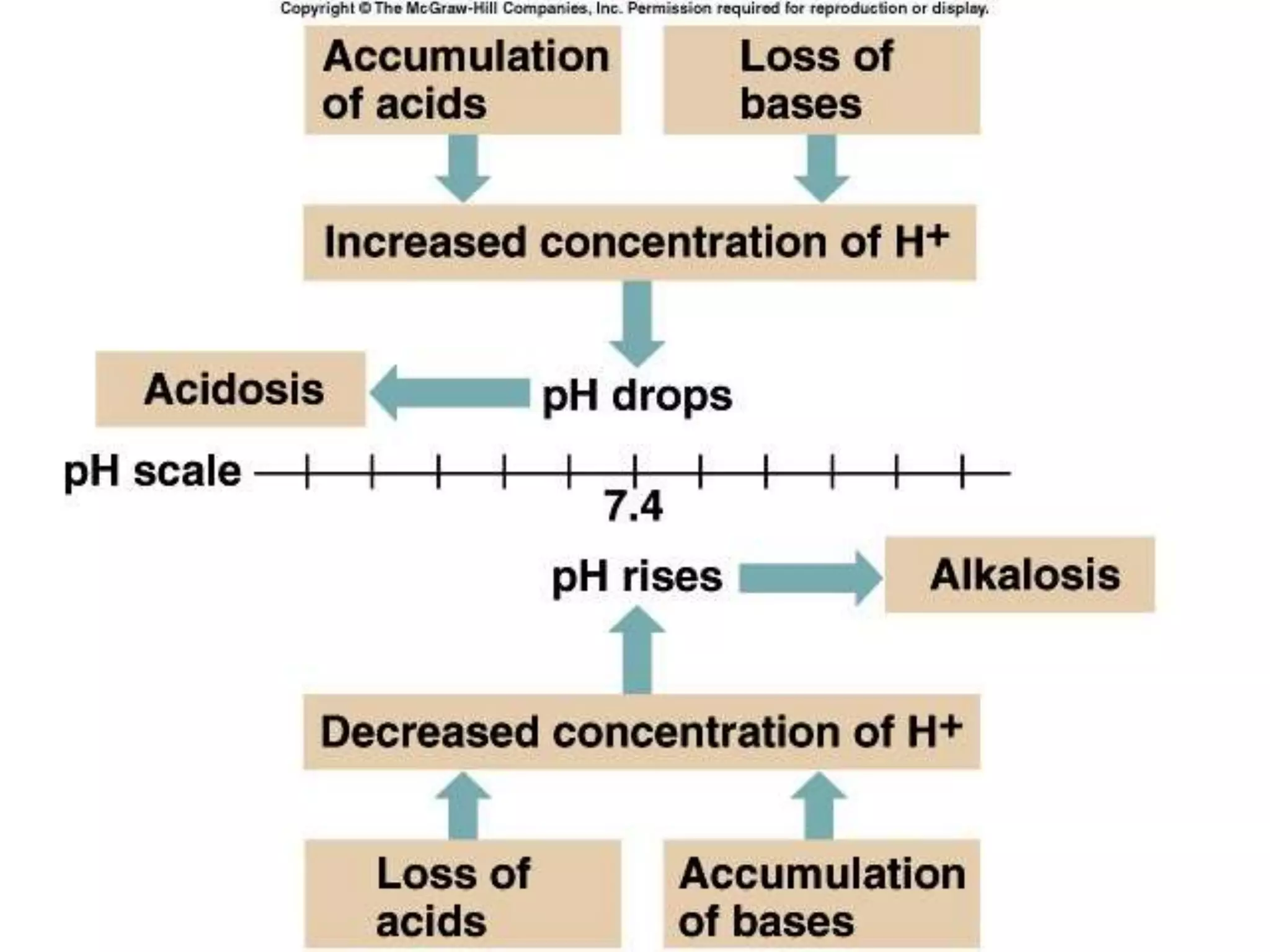
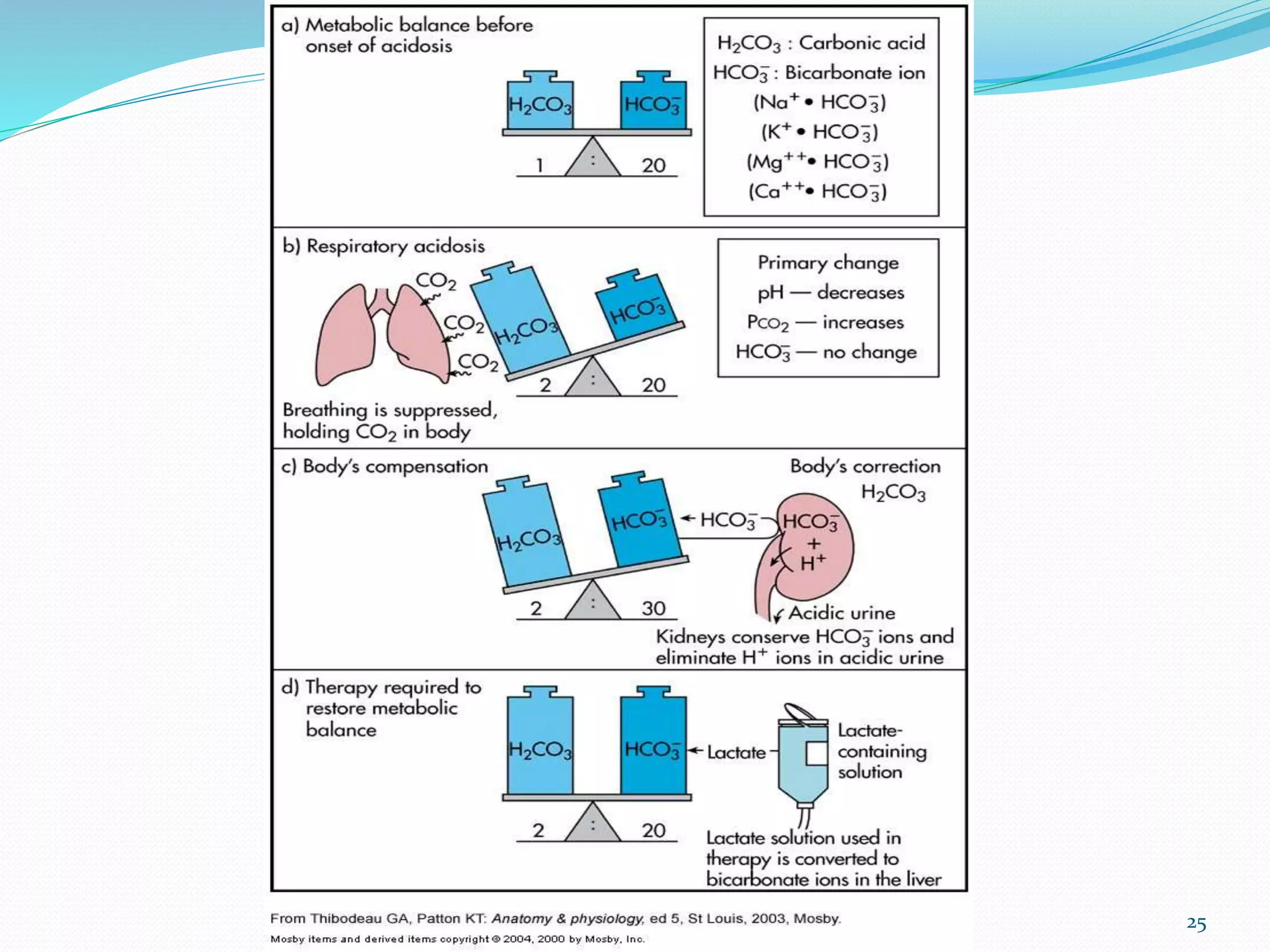
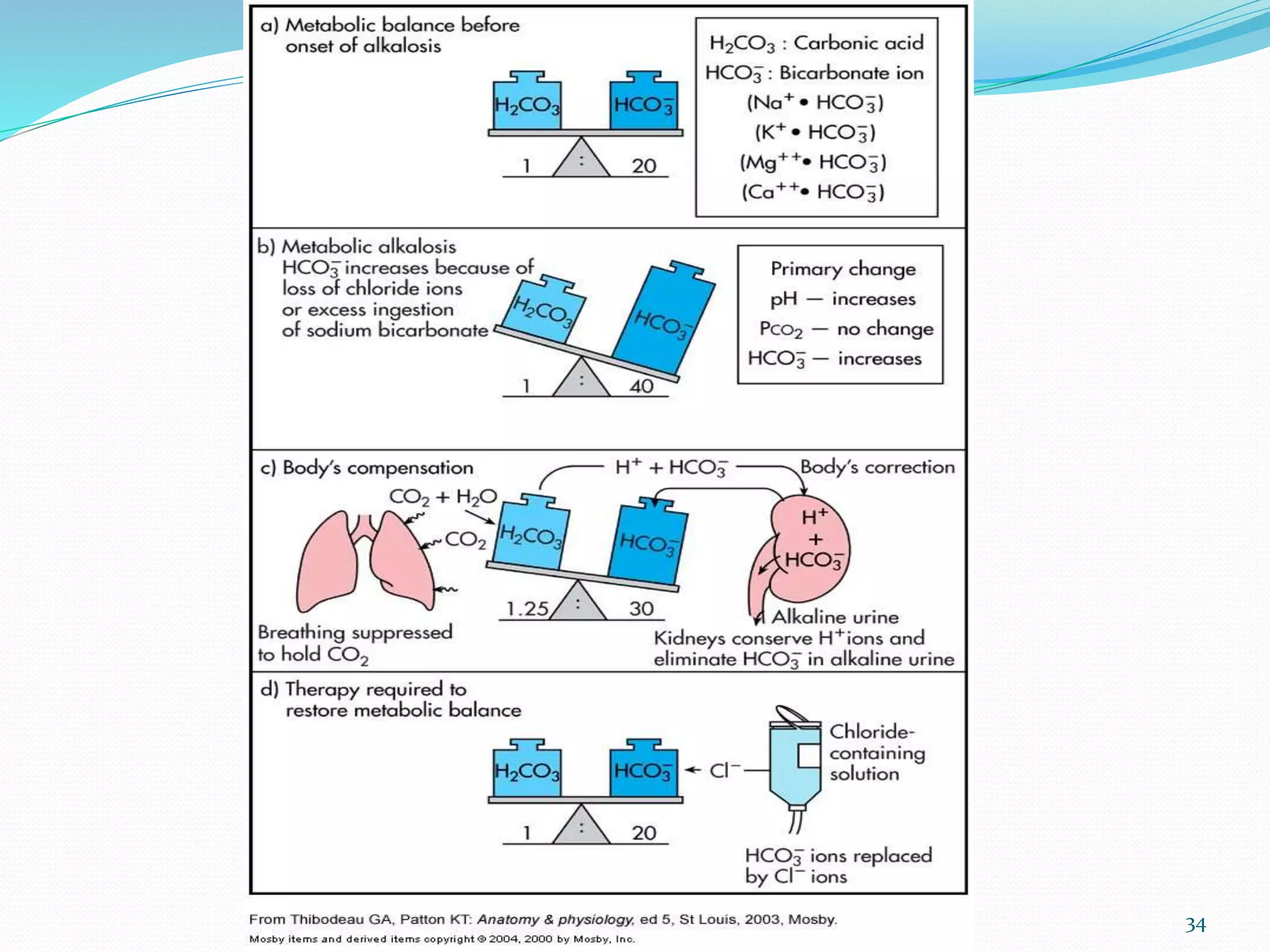
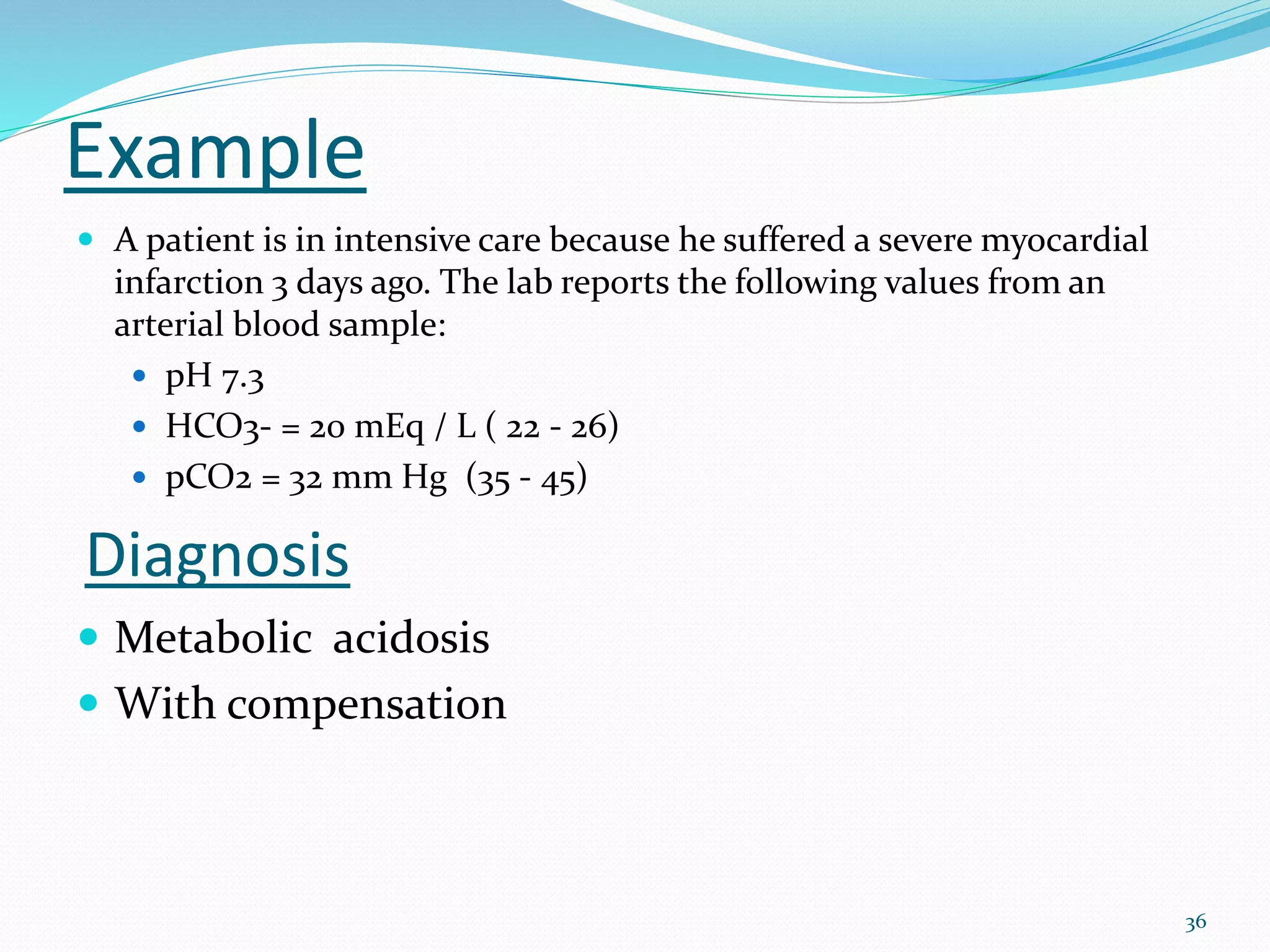
This document provides an overview of acid-base balance and pH regulation in the body. It defines pH and the scales used to measure acidity and alkalinity. It describes how the body tightly controls pH through buffer systems, respiration, and kidney function. Disruptions in acid-base balance can cause metabolic acidosis, alkalosis, respiratory acidosis or alkalosis. The document outlines signs, symptoms, causes, and treatments for different acid-base imbalances. It also provides examples of interpreting arterial blood gas results to diagnose specific acid-base disorders.

![2
pH Review
pH = - log [H+]
H+ is really a proton
Range is from 0 - 14
If [H+] is high, the solution is acidic; pH < 7
If [H+] is low, the solution is basic or alkaline ; pH > 7](https://image.slidesharecdn.com/acidbasebalance-200413102143/75/Acid-and-Base-Balance-and-Imbalance-2-2048.jpg)






![Henderson-Hasselbach equation
pH = pKa + log([HCO3
-]/[H2CO3])
CO2 dissolved in plasma potentially can form H2CO3 so:
pH = pKa + log([HCO3
-]/[dissolved CO2 + H2CO3])
At the temperature and ionic strength of ECF there are 6,800
HCO3
- ions and 340 molecules of dissolved CO2 for each
molecule of H2CO3. Therefore, H2CO3 can be neglected
Dissolved CO2 is related to pCO2 by its solubility coefficient:
Dissolved CO2 = 0.03 X pCO2](https://image.slidesharecdn.com/acidbasebalance-200413102143/75/Acid-and-Base-Balance-and-Imbalance-9-2048.jpg)
![Henderson-Hasselbach equation (clinically
relevant form)
pH = pKa + log([HCO3
-]/.03xpCO2)
pH = 6.1 + log([HCO3
-]/.03xpCO2)
Shows that pH is a function of the RATIO
between bicarbonate and pCO2](https://image.slidesharecdn.com/acidbasebalance-200413102143/75/Acid-and-Base-Balance-and-Imbalance-10-2048.jpg)