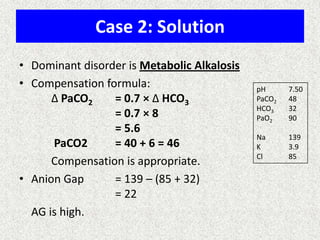
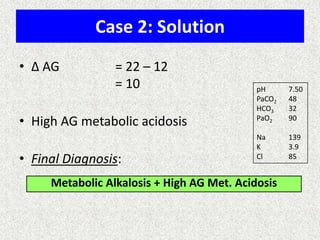
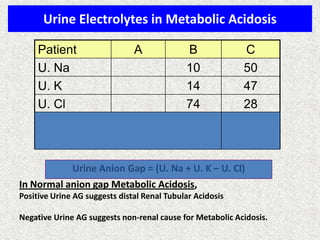
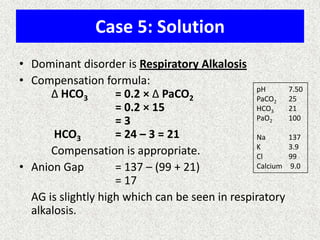
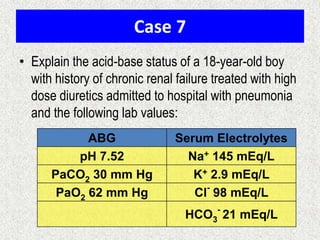
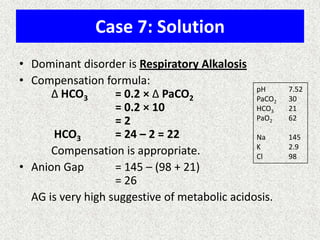
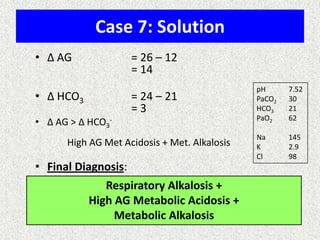
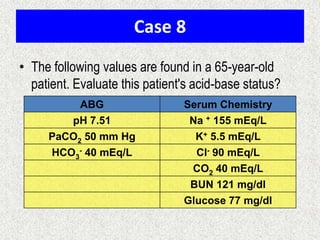
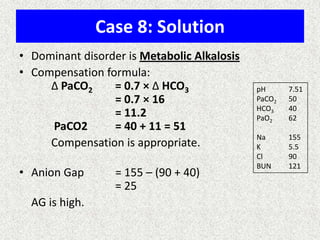
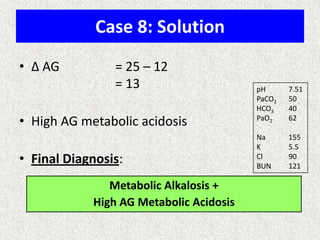
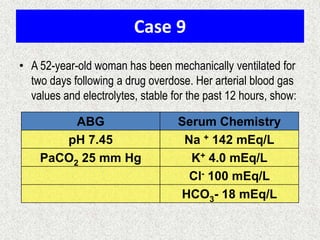
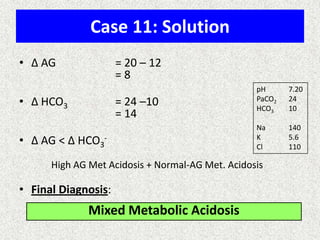
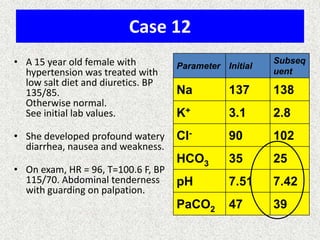
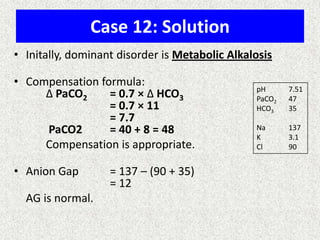
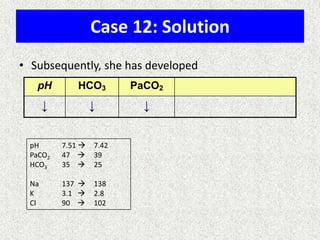
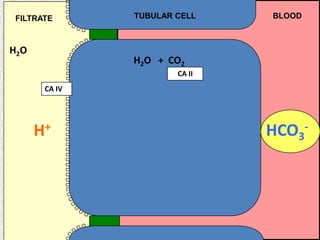
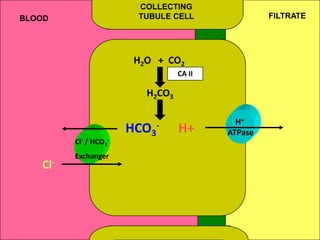
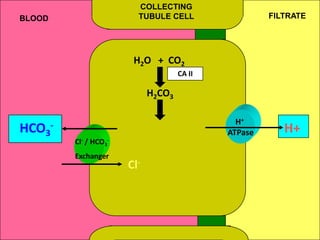
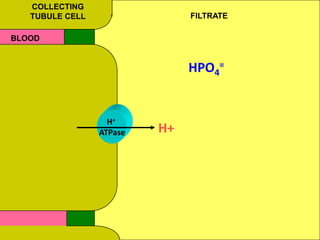
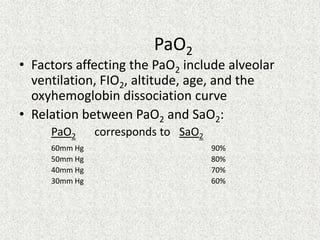
1) The document discusses approaches to analyzing blood gases and acid-base disorders. It provides details on how the kidney regulates acid-base balance through bicarbonate reabsorption and secretion of hydrogen ions. Formulas for calculating compensation and identifying dominant acid-base disorders are presented.
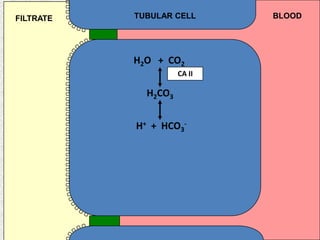
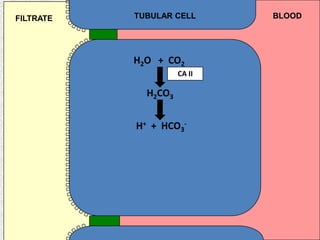
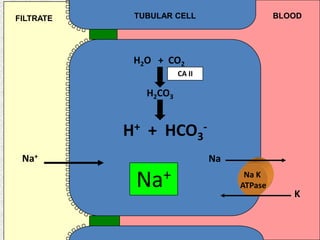
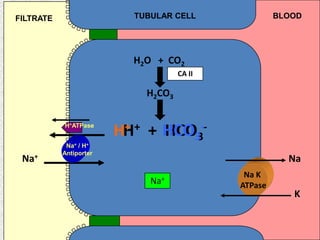
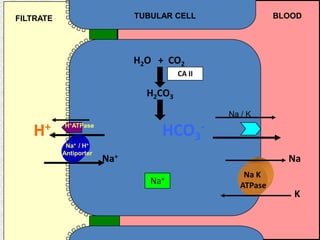
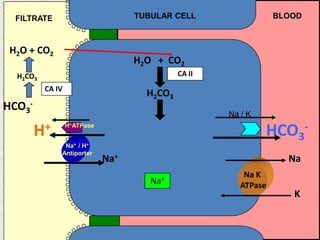
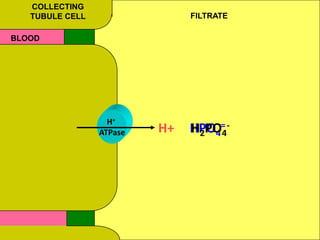
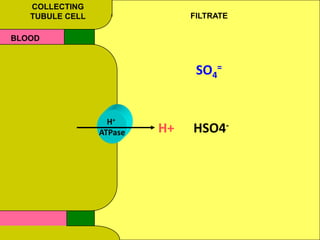
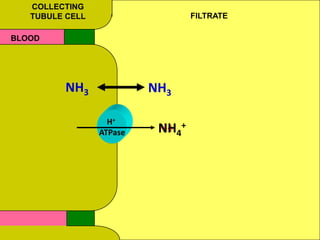
2) Mechanisms of bicarbonate and hydrogen ion transport across renal tubular cells are illustrated through diagrams. Equations for calculating expected compensation in common acid-base imbalances are given to help identify the primary disorder.
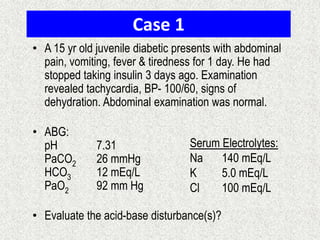
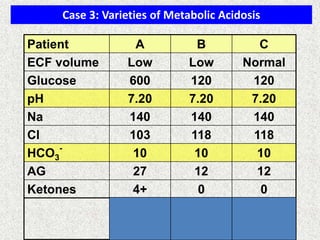
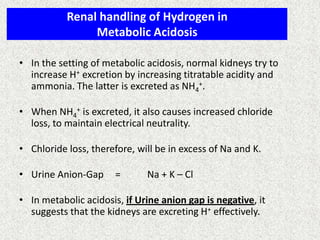
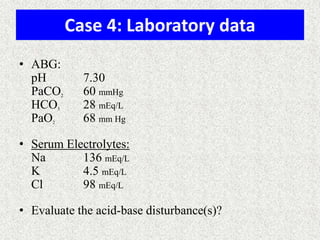
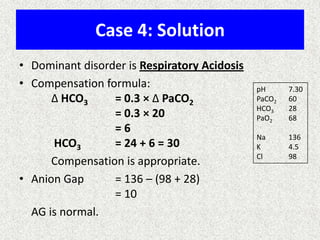
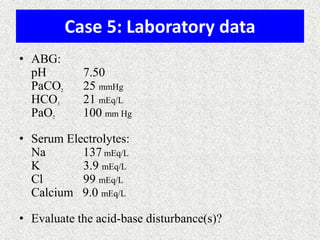
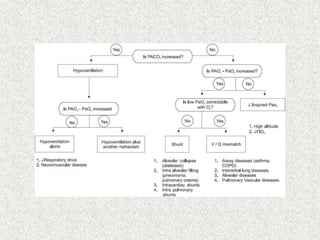
3) Methods for evaluating systemic acid-base disorders are outlined, including using arterial blood gas results and serum electrolytes to identify


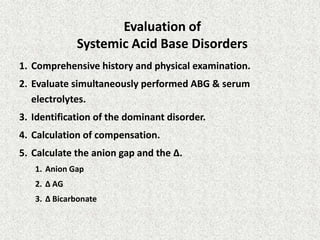

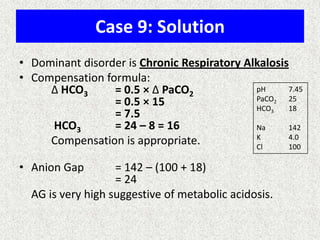
![Calculation of compensation
Mean "whole body" response equations for simple acid-base disturbances.
Note: The formula calculates the change in the compensatory parameter.
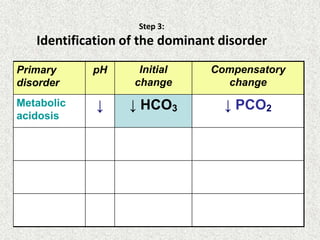
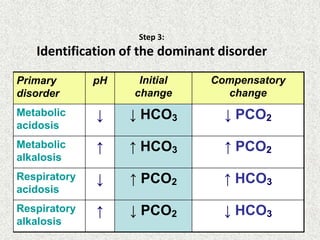
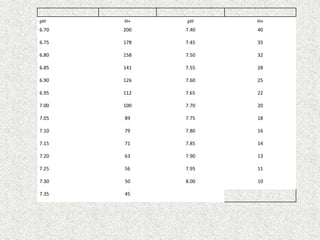
Disorder pH Primary
change
Compensatory
Response
Equation
Metabolic
Acidosis
[HCO3
-] PCO2 ΔPCO2 1.2 ΔHCO3
Metabolic
Alkalosis
[HCO3
-] PCO2 ΔPCO2 0.7 ΔHCO3
Respiratory
Acidosis
PCO2 [HCO3
-] Acute:
ΔHCO3
- 0.1 ΔPCO2
Chronic:
ΔHCO3
- 0.3 ΔPCO2
Respiratory
Alkalosis
PCO2 [HCO3
-] Acute:
ΔHCO3
- 0.2 ΔPCO2
Chronic:
ΔHCO3
- 0.5 ΔPCO2](https://image.slidesharecdn.com/presentation1-140316021754-phpapp01/85/DNB-OSCE-ON-ABG-23-320.jpg)
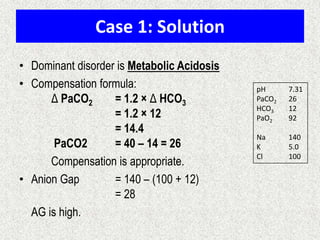
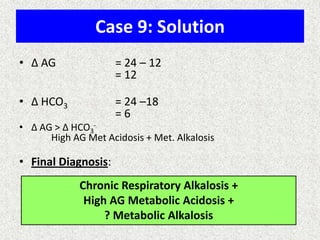
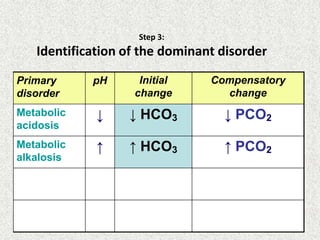
![Simple compensation
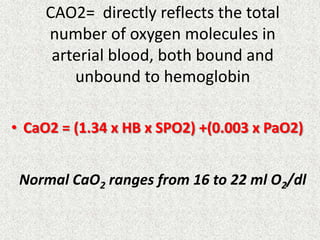
Disorder pH Primary problem Compensation
Metabolic acidosis ↓ ↓ in HCO3- PaCO2
=1.5xHCO3+8(+/-2)
Metabolic alkalosis ↑ 10↑ in HCO3- 7↑ in PaCO2
Respiratory acidosis ↓ ACUTE -10↑ in PaCO2
CHRONIC -10↑ in PaCO2
1↑ in [HCO3-]
3.5↑ in [HCO3-]
Respiratory alkalosis ↑ ACUTE-10↓ in PaCO2
CHRONIC-10↓ in PaCO2
2↓ in [HCO3-]
4↓ in [HCO3-]](https://image.slidesharecdn.com/presentation1-140316021754-phpapp01/85/DNB-OSCE-ON-ABG-24-320.jpg)
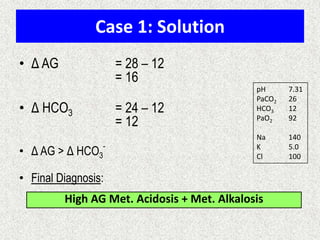
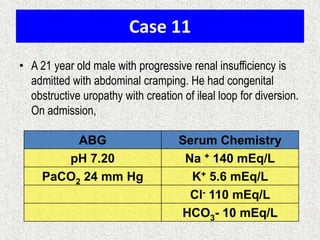
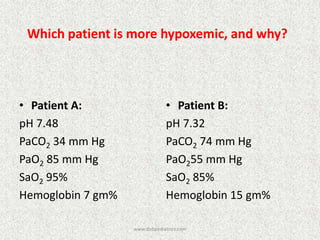
![Calculate the “gaps”
Anion gap = Na+ − [Cl− + HCO3
−]
Δ AG = Anion gap − 12
Δ HCO3 = 24 − HCO3
Δ AG = Δ HCO3
−, then Pure high AG Met. Acidosis
Δ AG > Δ HCO3
−, then High AG Met Acidosis + Met. Alkalosis
Δ AG < Δ HCO3
−, then High AG Met Acidosis + Normal AG Met A
Note:
Add Δ AG to measured HCO3
− to obtain bicarbonate level
that would have existed IF the high AG metabolic acidosis
were to be absent, i.e., “Pre-existing Bicarbonate.”
Bicarbexistinge
BicarbCurrent
AGDelta
__Pr
_
_
](https://image.slidesharecdn.com/presentation1-140316021754-phpapp01/85/DNB-OSCE-ON-ABG-25-320.jpg)

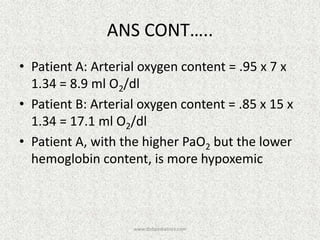


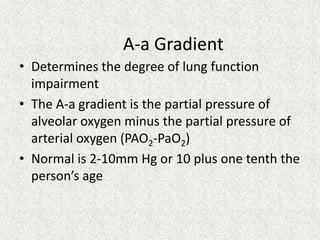
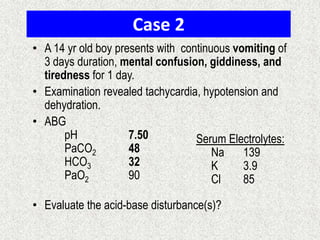
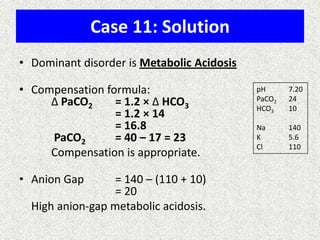
![A-a Gradient
• [(713*FIO2)-(PaCO2/0.8)] – PaO2
INTERPRETATION
NORMAL – 10-20
(>30 is SINGNIFICANT)
Seen in – Shunt
Low V/Q
Hypoventilation](https://image.slidesharecdn.com/presentation1-140316021754-phpapp01/85/DNB-OSCE-ON-ABG-36-320.jpg)


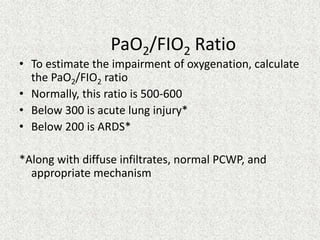



![RELATION OF ALBUMIN IN ABG
AG corrected = AG + 2.5[4 – albumin]
(AG= Anion gap)](https://image.slidesharecdn.com/presentation1-140316021754-phpapp01/85/DNB-OSCE-ON-ABG-44-320.jpg)