This document provides a summary of acid-base physiology, including:
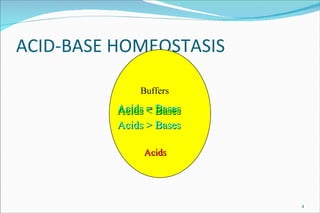
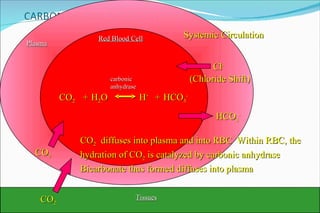
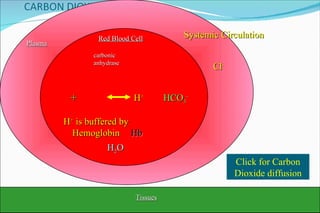
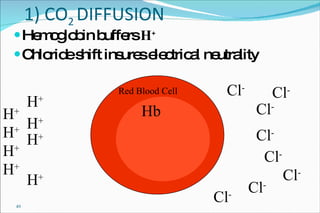
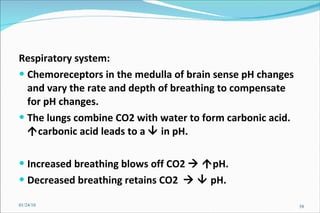
1) Homeostatic mechanisms that regulate acid-base balance, including chemical buffers, respiratory regulation, and renal regulation.
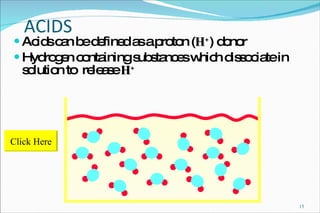
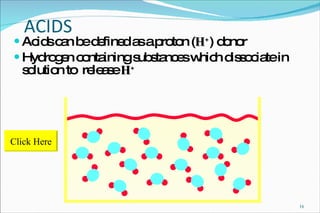
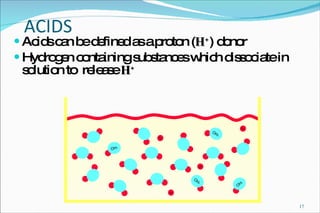
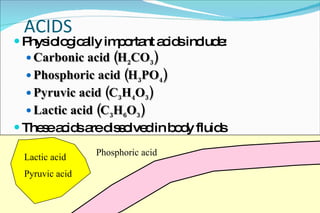
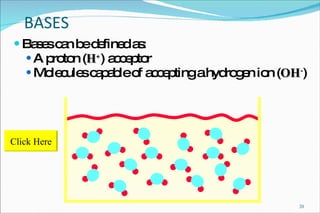
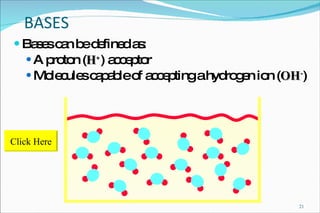
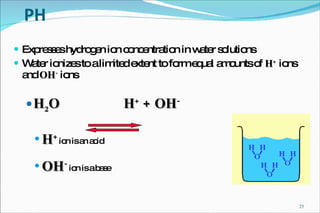
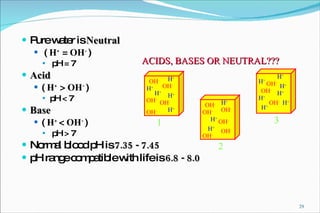
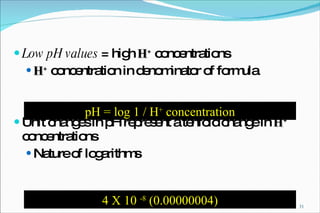
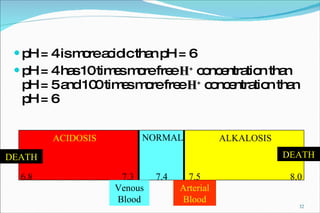
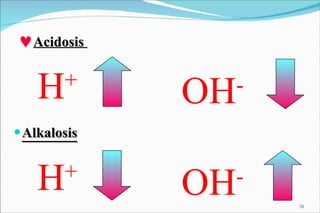
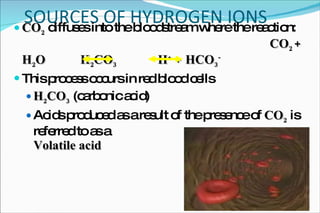
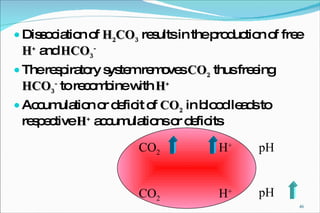
2) Definitions of acids, bases, and the pH scale. Acidosis and alkalosis can arise from excess or deficits of volatile or fixed acids.
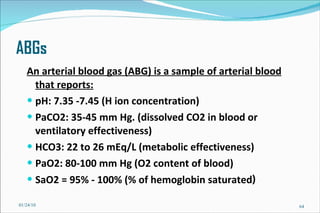
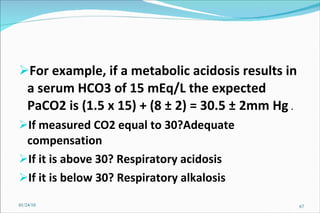
3) Key concepts in acid-base regulation including the Henderson-Hasselbalch equation and analyzing arterial blood gases.

















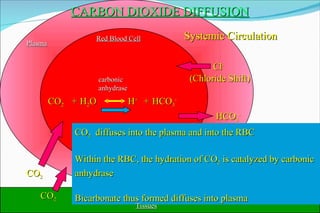
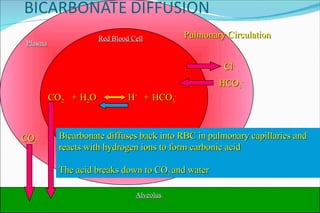
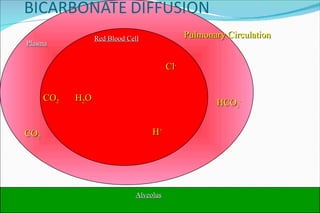
![Basic Concepts The hydrogen ion concentration [H+] in extra cellular fluid is determined by the balance between the partial pressure of carbon dioxide (PCO2)/HCO3 in the fluid. This relationship is expressed as follows : [H+] (nEq/L) = 24 x (PCO2/HCO3) 01/24/10](https://image.slidesharecdn.com/acid-base-balance4818/85/Acid-Base-Balance-53-320.jpg)
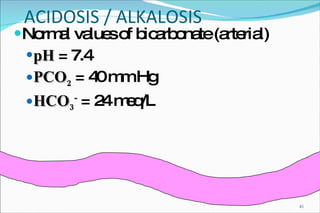
![Using a normal arterial PCO2 of 40 mm Hg and a normal serum HCO3 concentration of 24 mEq/L, the normal [H+] in arterial blood is 24 x (40/24) = 40 nEq/L. 01/24/10](https://image.slidesharecdn.com/acid-base-balance4818/85/Acid-Base-Balance-54-320.jpg)





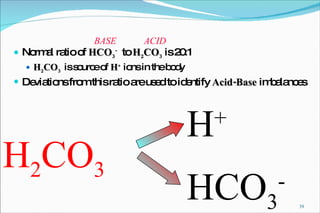
![Henderson-Hasselbalch Equation pH = pK a + log [base]/[acid] Ex: = 6.1 + log 20/1 = 6.1 + 1.3 = 7.4 Key ratio is base: acid HCO 3 - : CO 2 (standing in for H 2 CO 3 )](https://image.slidesharecdn.com/acid-base-balance4818/85/Acid-Base-Balance-63-320.jpg)




![Compensation Acute Respiratory Δ pH = 0.008 x Δ PaCO2 Acidosis Expected pH = 7.40 –[0.008x(PaCO2 -40)] Chronic Respiratory Δ pH = 0.003 x Δ PaCO2 Acidosis Expected pH = 7.40 –[0.003x(PaCO2 -40)] Acute Respiratory Δ pH = 0.008 x Δ PaCO2 Alkalosis Expected pH = 7.40 + [0.008 x (40-PaCO2)] Chronic Respiratory Δ pH = 0.003 x Δ PaCO2 Alkalosis Expected pH = 7.40 + [0.003 x (40-PaCO2)] 01/24/10](https://image.slidesharecdn.com/acid-base-balance4818/85/Acid-Base-Balance-70-320.jpg)



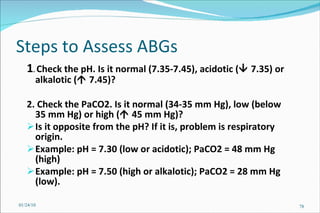
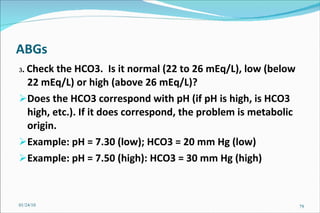

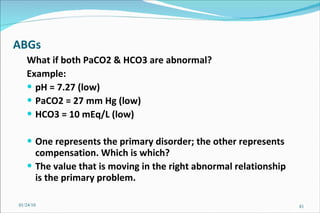
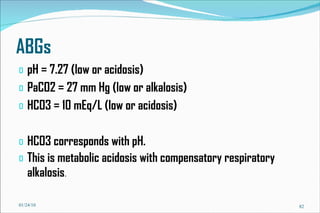


![Problem Consider a hypothetical situation in which the PCO2 is 80 mmHg and [HCO3] 48 mM of arterial plasma. Which of the following statements is correct? A . There is no acid-base disturbance. B . Metabolic alkalosis is the primary acid-base disturbance. C . Respiratory acidosis is the primary acid-base disturbance. D . Intracellular pH is lower than normal. E . Intracellular pH is higher than normal. 01/24/10](https://image.slidesharecdn.com/acid-base-balance4818/85/Acid-Base-Balance-85-320.jpg)
