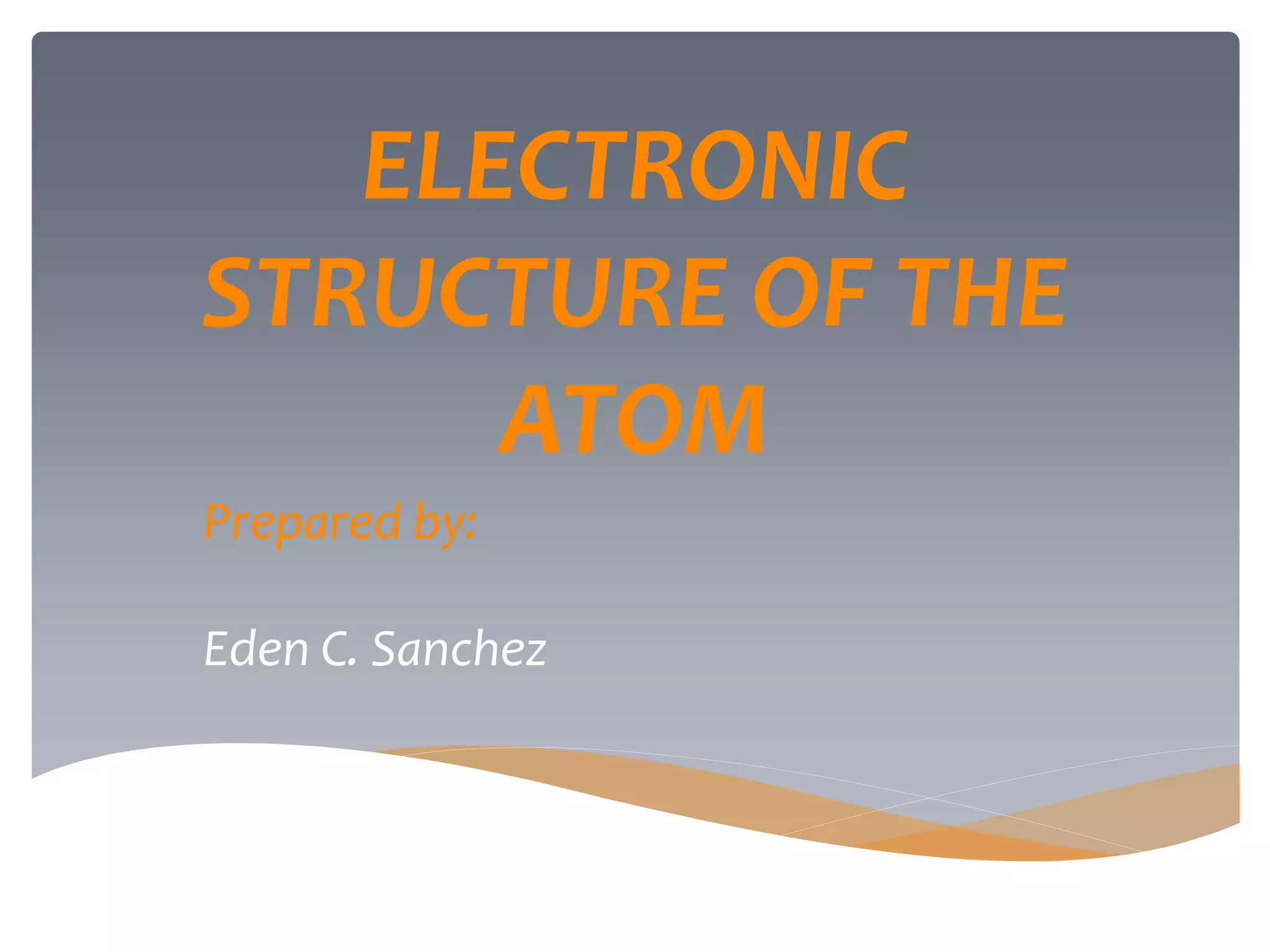
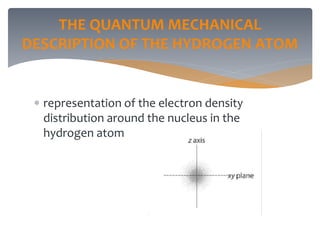
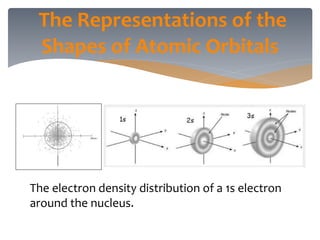
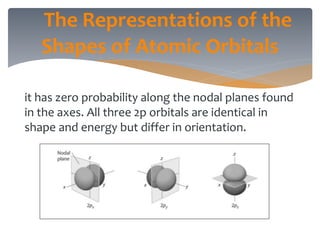
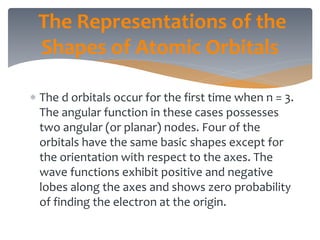
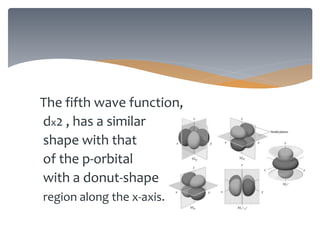
The document discusses the electronic structure of the atom, focusing on Heisenberg's uncertainty principle and the Schrodinger equation, which describes electron behavior and atomic orbitals. It explains how quantum numbers characterize orbitals, detailing their roles in determining energy, shape, and orientation of electrons. Additionally, it outlines the shapes of atomic orbitals, illustrating the probability distribution of electrons and their associated densities.