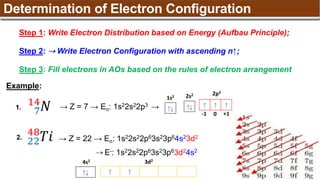
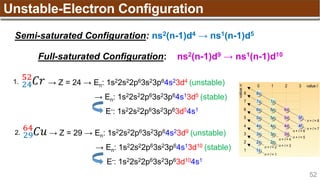
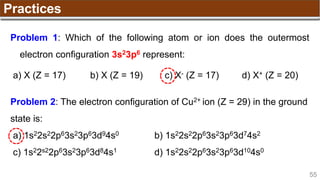
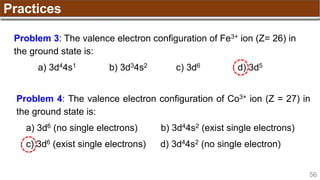
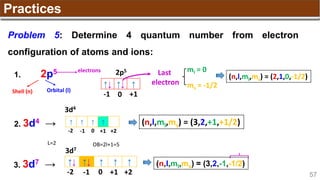
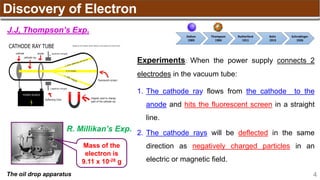
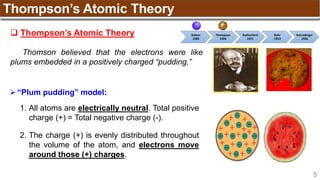
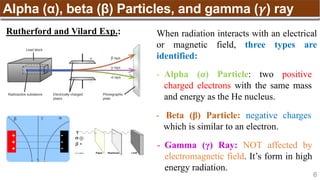
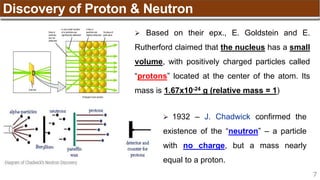
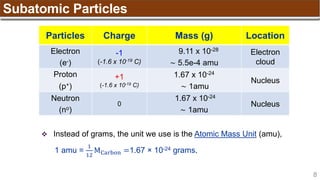
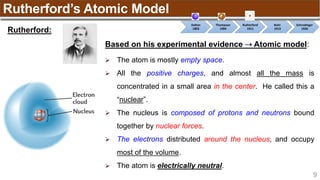
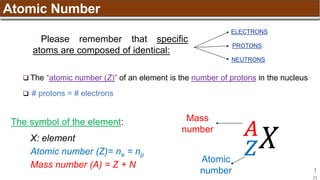
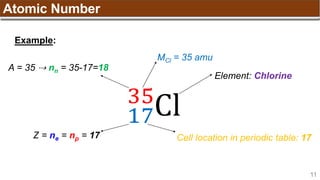
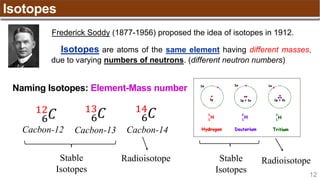
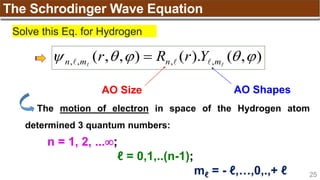
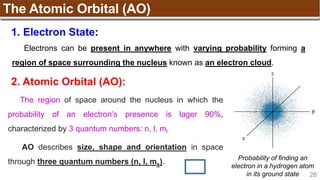
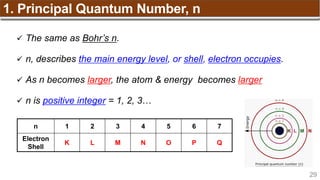
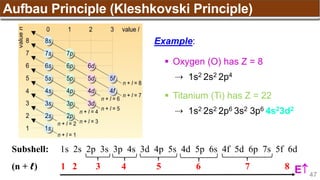
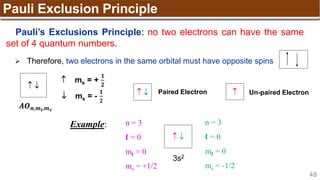
The document provides an overview of atomic structure, detailing historical developments in atomic theory from Dalton to Schrödinger and discussing key concepts such as atomic models, subatomic particles, quantum numbers, and the Schrödinger wave equation. It outlines significant findings related to atomic particles including electrons, protons, and neutrons, and explains various models including Bohr's model and the modern quantum mechanical model. Additionally, it touches on wave-particle duality and the significance of quantum numbers in determining the state of electrons in an atom.




![49
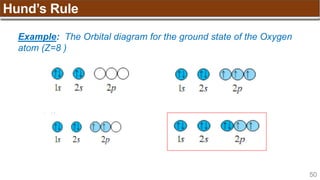
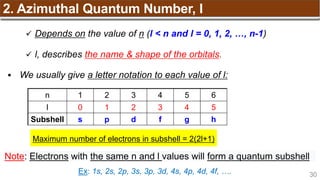
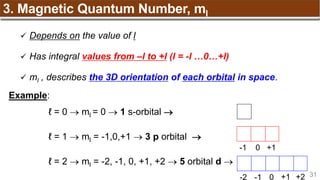
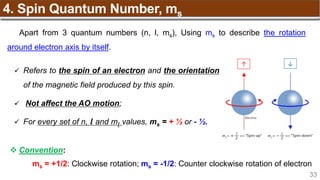
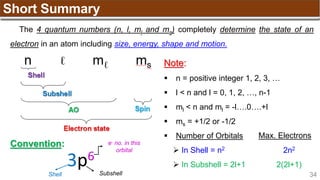
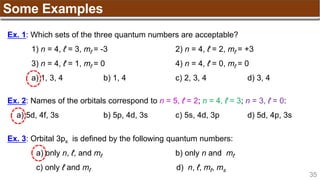
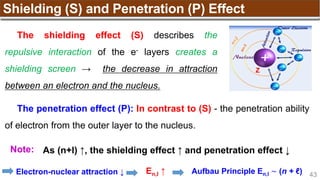
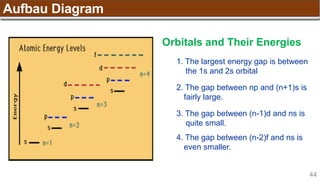
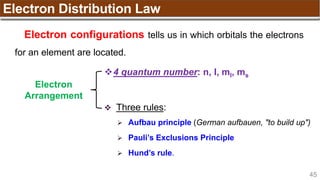
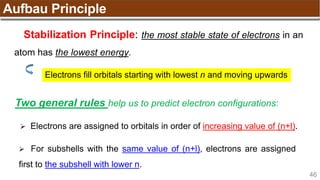
Hund’s Rule
Hund’s Rule: electrons in the same subshell must be
distributed so that the absolute value of the total spin or unpair
electrons is maximized
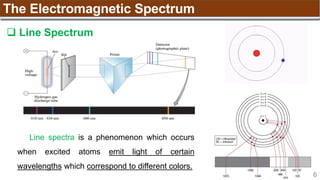
Electrons fill each orbital singly before any orbital gets a second electron
6
12
C → Z = 6 → 1s22s22p2 → ↑↓
1s2
↑↓
2s2 ↑↓
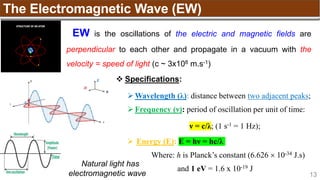
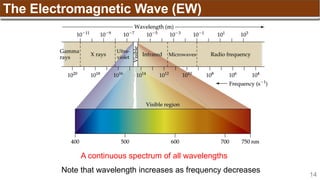
2p2
↑ ↑
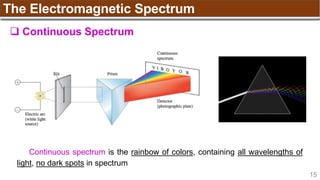
-1 0 +1
-1 0 +1
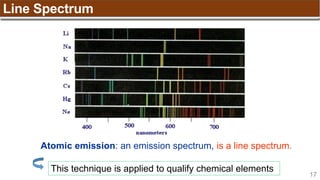
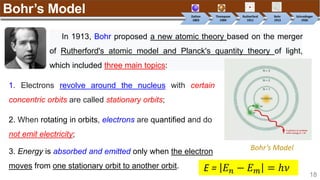
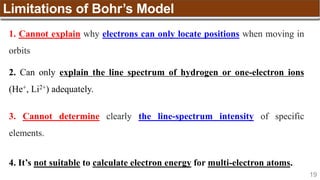
Example:
[Trong phân lớp, điền spin dương (↑) đủ các obitals sau đó điền spin âm (↓)]
Nitrogen (N) has Z = 7 ➡
1s 2s 2p](https://image.slidesharecdn.com/chapter1atomicstructurepdf-240722073243-9bff3ed6/85/Chapter-1_Atomic-Structure_PDF_GENERAL-CHEMISTRY-49-320.jpg)