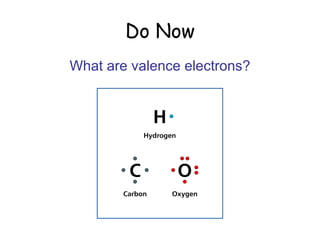
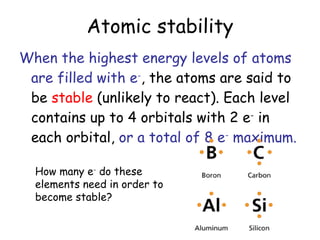
The document discusses valence electrons and bonding. It introduces the 2-8-8 rule which states that the first energy level can hold up to 2 electrons, and the second and third levels can each hold up to 8 electrons. Atoms are stable once their energy levels are filled. Ionic bonds form when a metal atom transfers electrons to a nonmetal, becoming cations and anions. Covalent bonds form when atoms share electrons rather than transfer them. Metallic bonds form a "sea" of delocalized electrons between positively charged metal ions.