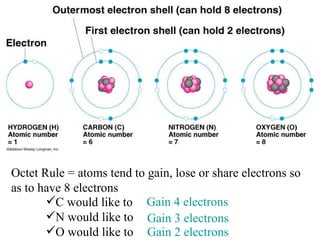
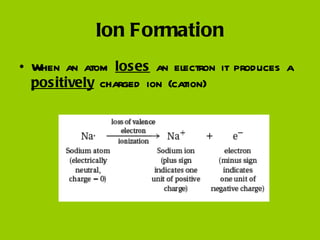
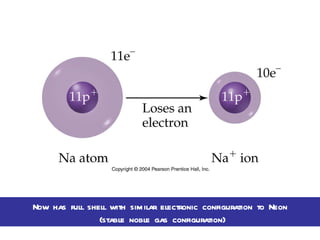
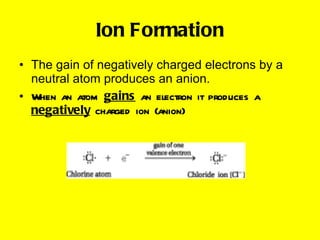
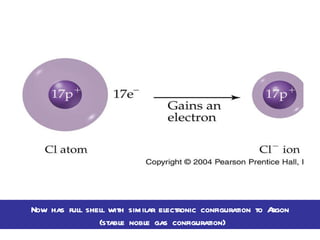
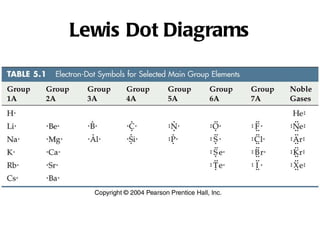
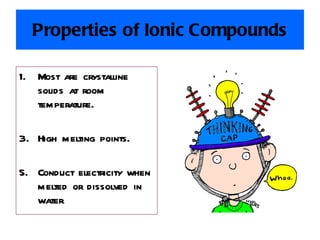
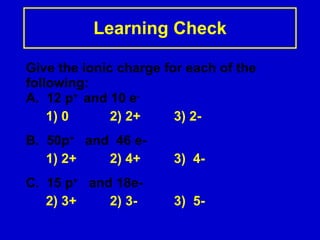
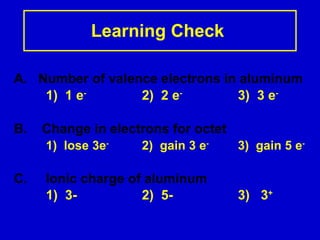
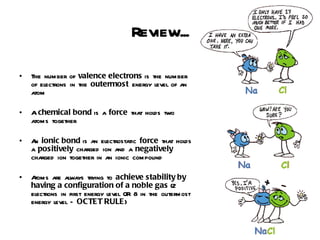
Ionic bonding occurs when atoms gain or lose valence electrons to achieve stable noble gas configurations, becoming ions. Cations are positively charged ions formed when atoms lose electrons, while anions are negatively charged ions formed when atoms gain electrons. Ionic compounds are formed from electrostatic attractions between cations and anions, resulting in electrically neutral compounds. Ionic compounds usually have high melting points and conduct electricity when molten or dissolved in water.