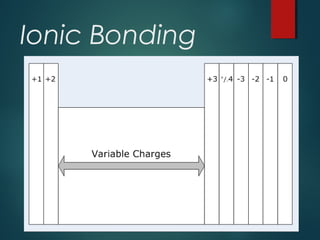
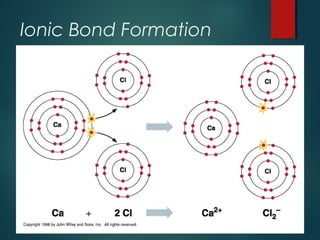
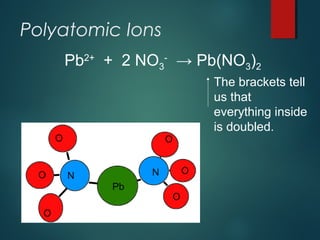
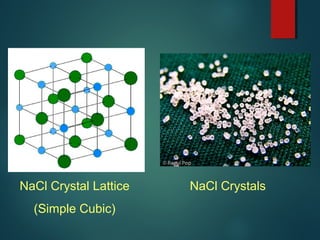
Ionic compounds are formed through ionic bonding between metals and nonmetals. Electrons are transferred from the metal atoms to the nonmetal atoms, resulting in cations with positive charges and anions with negative charges. The electrostatic forces between the oppositely charged ions hold the compound together in a crystalline lattice structure. Common polyatomic ions like nitrate, sulfate, and phosphate are also present in ionic compounds. Ionic compounds have properties like being solid at room temperature, having high melting points, and being good conductors of electricity when molten or dissolved in water.