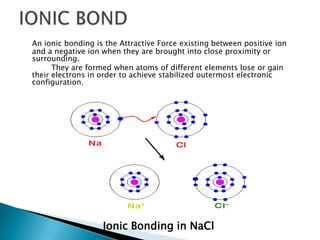
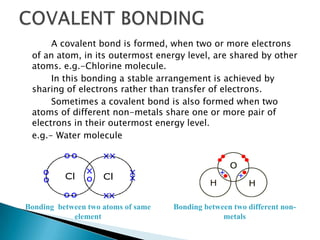
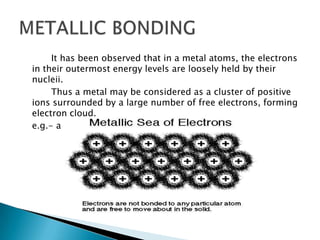
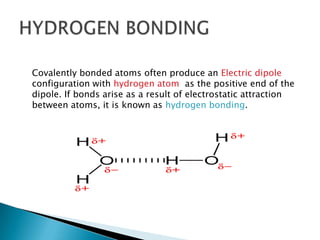
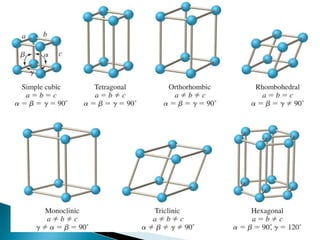
Ionic, covalent, metallic, hydrogen, and Van Der Wall's bonding are the main types of bonding. Ionic bonding occurs between positive and negative ions, covalent bonding involves sharing electrons between atoms, and metallic bonding involves loosely bound electrons in metal atoms that form an electron cloud. Hydrogen bonding arises from electrostatic attraction between hydrogen atoms partially charged by covalent bonds. Van Der Wall's bonding involves weak, temporary bonds between molecules of the same substance.