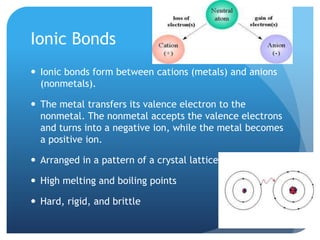
This document discusses the three main types of chemical bonds: ionic, covalent, and metallic. Ionic bonds form between metals and nonmetals when the metal transfers electrons to the nonmetal. Covalent bonds form when atoms share electrons in either single, double or triple bonds. Metallic bonds form when metal atoms contribute electrons to form a "sea of electrons" that are shared between all the atoms.