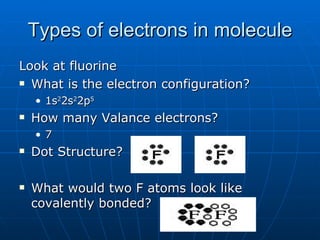
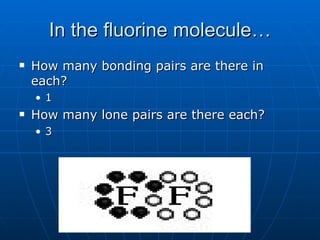
The document summarizes key concepts about covalent bonds, including how they form through the sharing of valence electrons between atoms to achieve stability. It discusses ionic vs. covalent bonds, how covalent bonds are represented using Lewis dot structures, and how the octet rule determines bonding patterns for atoms in different groups on the periodic table. Examples of writing Lewis dot structures for several diatomic and polyatomic molecules are provided.