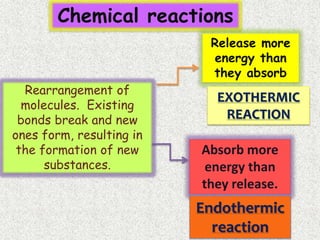
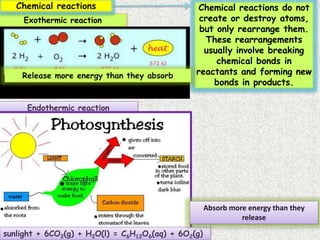
The document discusses different types of chemical bonds:
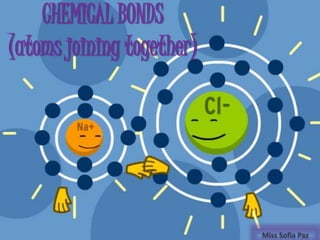
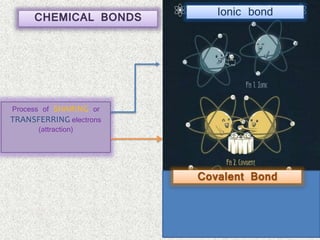
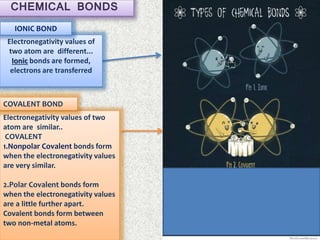
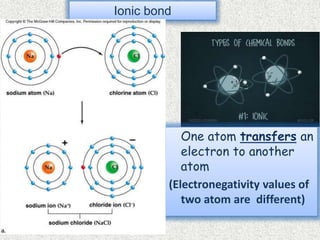
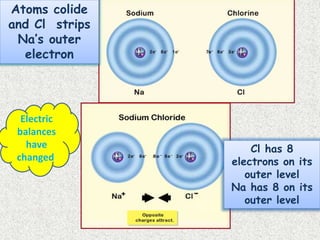
- Ionic bonds form when there is a large difference in electronegativity between two atoms, causing one atom to transfer an electron to the other.
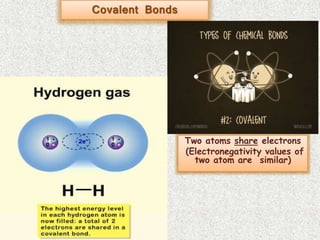
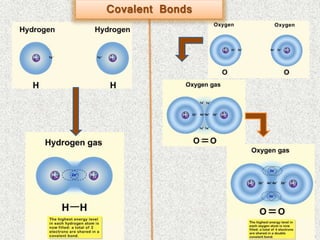
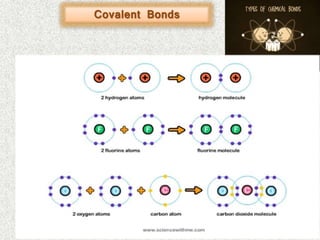
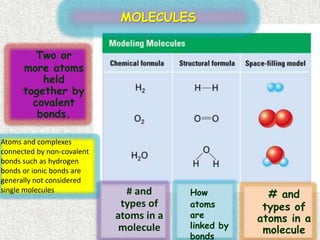
- Covalent bonds form when there is a small difference in electronegativity between two non-metal atoms, causing them to share electrons. Covalent bonds can be nonpolar or polar.
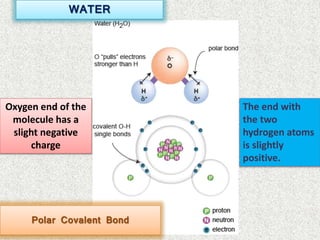
- Water is an example of a polar covalent molecule, with the oxygen end being partially negative and the hydrogen ends being partially positive.