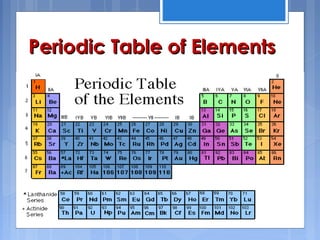
The periodic table is arranged based on atomic number and properties repeat periodically. Early forms grouped elements based on atomic mass but Moseley found atomic number was key. The table is divided into periods and groups with trends in properties like atomic size decreasing left to right across periods and ionization energy increasing. Metallic character increases down groups and nonmetallic character increases right across periods. The periodic table organizes the elements and allows prediction of properties.