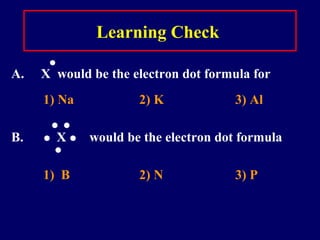
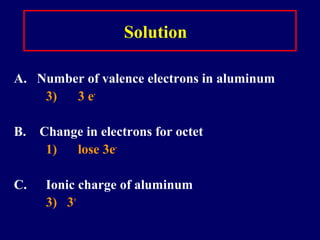
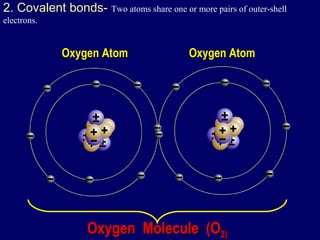
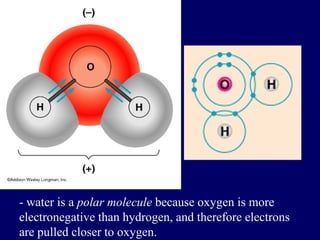
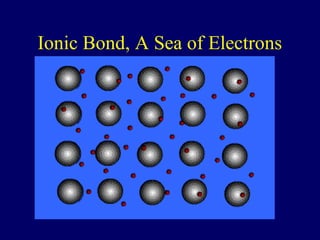
This document discusses the structure of atoms and the types of chemical bonds. It begins by defining the atom and its components like protons, electrons, and electron shells. It then explains the three main types of chemical bonds: ionic bonds formed between ions through electron transfer, covalent bonds formed by electron sharing, and metallic bonds in metals involving delocalized electrons. Some key points about each bond type are described, including examples. The document also touches on atomic and molecular masses and formula weights.