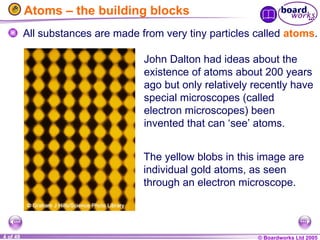
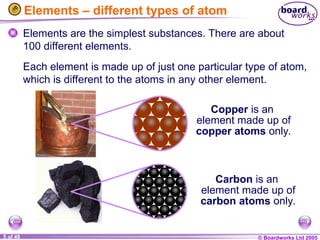
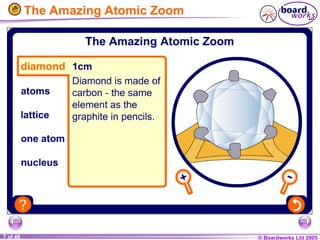
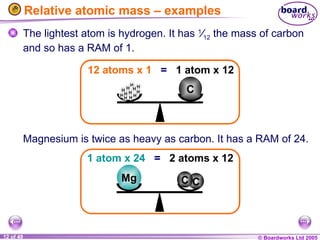
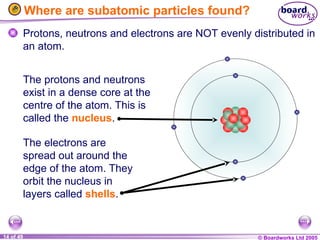
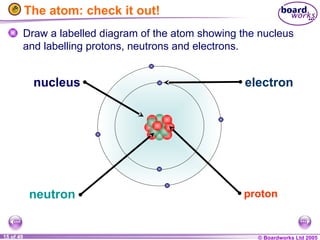
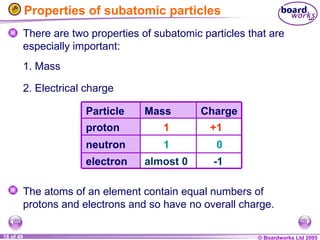
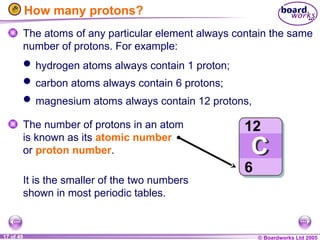
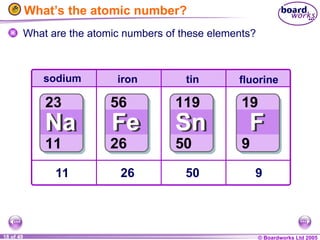
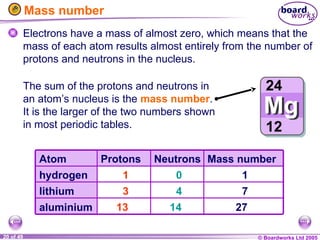
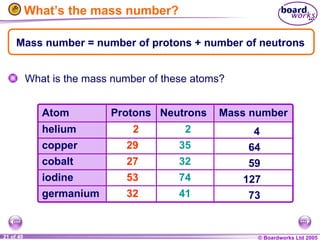
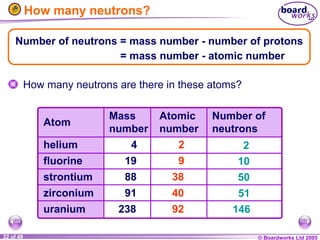
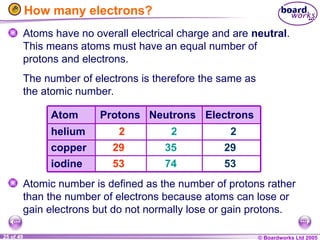
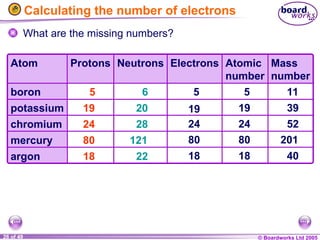
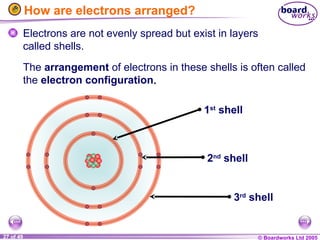
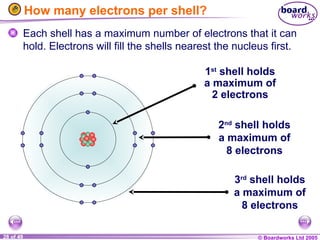
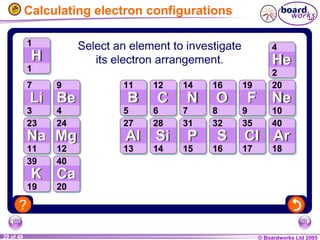
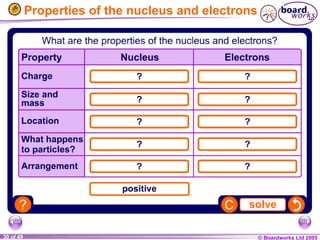
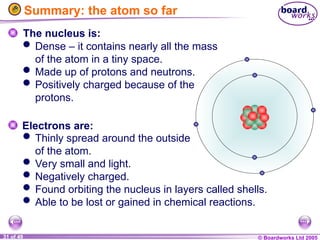
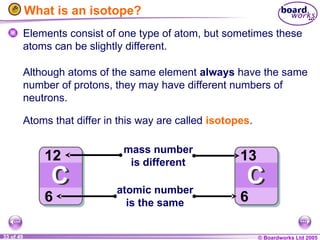
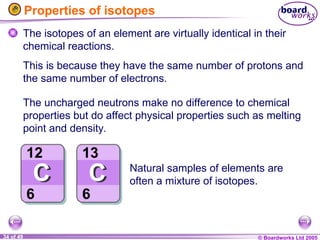
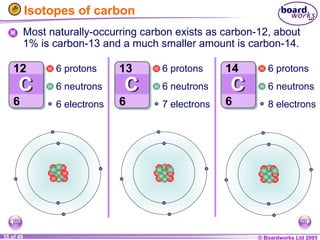
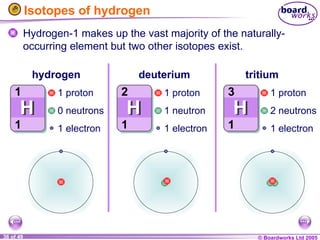
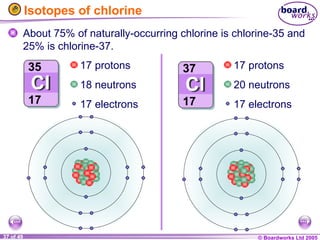
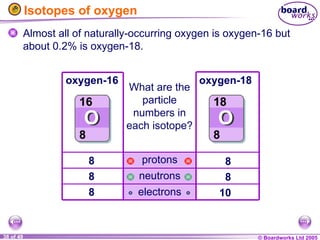
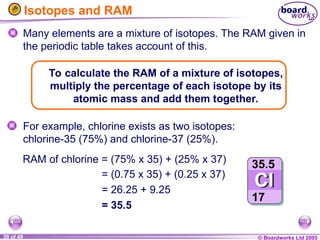
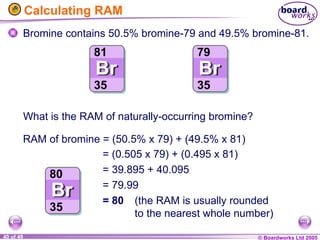
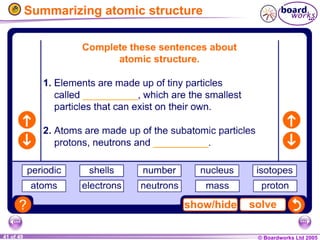
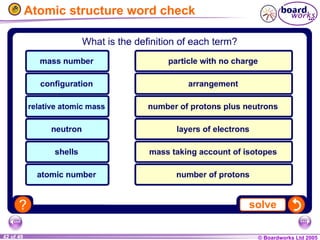
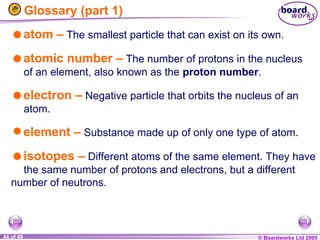
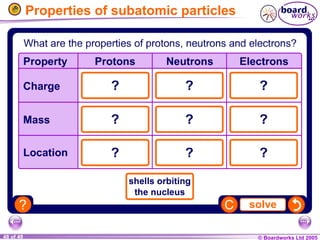
The document discusses atomic structure, including the existence of atoms, elements, and their properties. It explains protons, neutrons, and electrons, their arrangement within atoms, the concept of isotopes, and how the relative atomic mass is calculated. Additionally, it highlights the historical context of atomic theory and the techniques used to observe atoms.