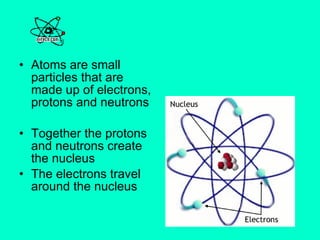
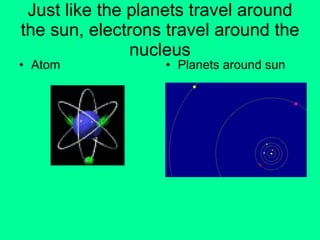
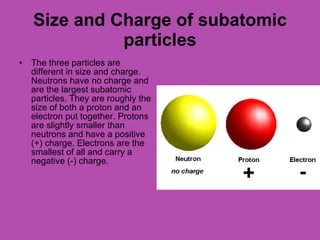
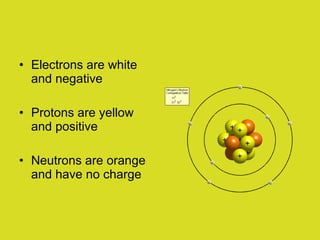
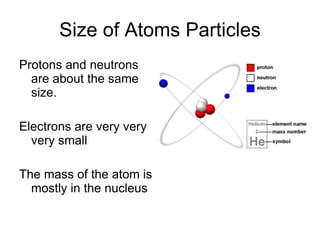
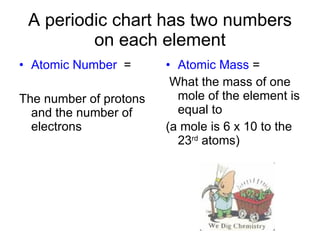
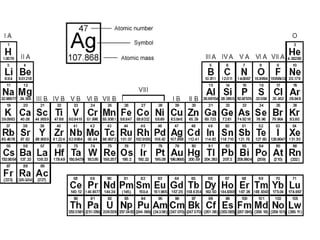
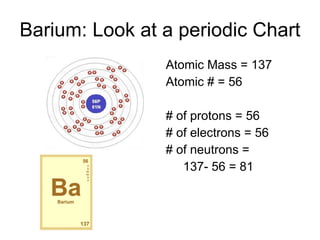
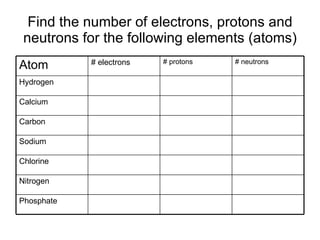
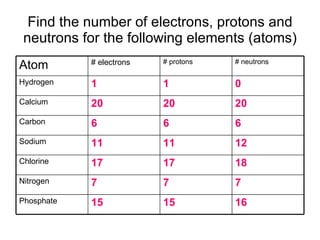
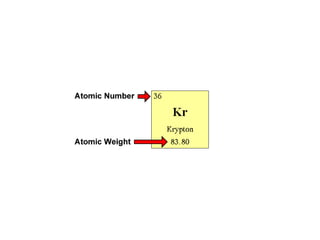
This document provides an overview of atoms and their subatomic particles. It explains that atoms are made up of protons, neutrons, and electrons. Protons have a positive charge, electrons have a negative charge, and neutrons have no charge. The number of protons determines the element, and the number of neutrons contributes to the mass number. The document also discusses the periodic table and how it can be used to find information about elements such as the number of protons, neutrons, and electrons. It includes some example calculations and provides additional online resources for learning more about atomic structure.