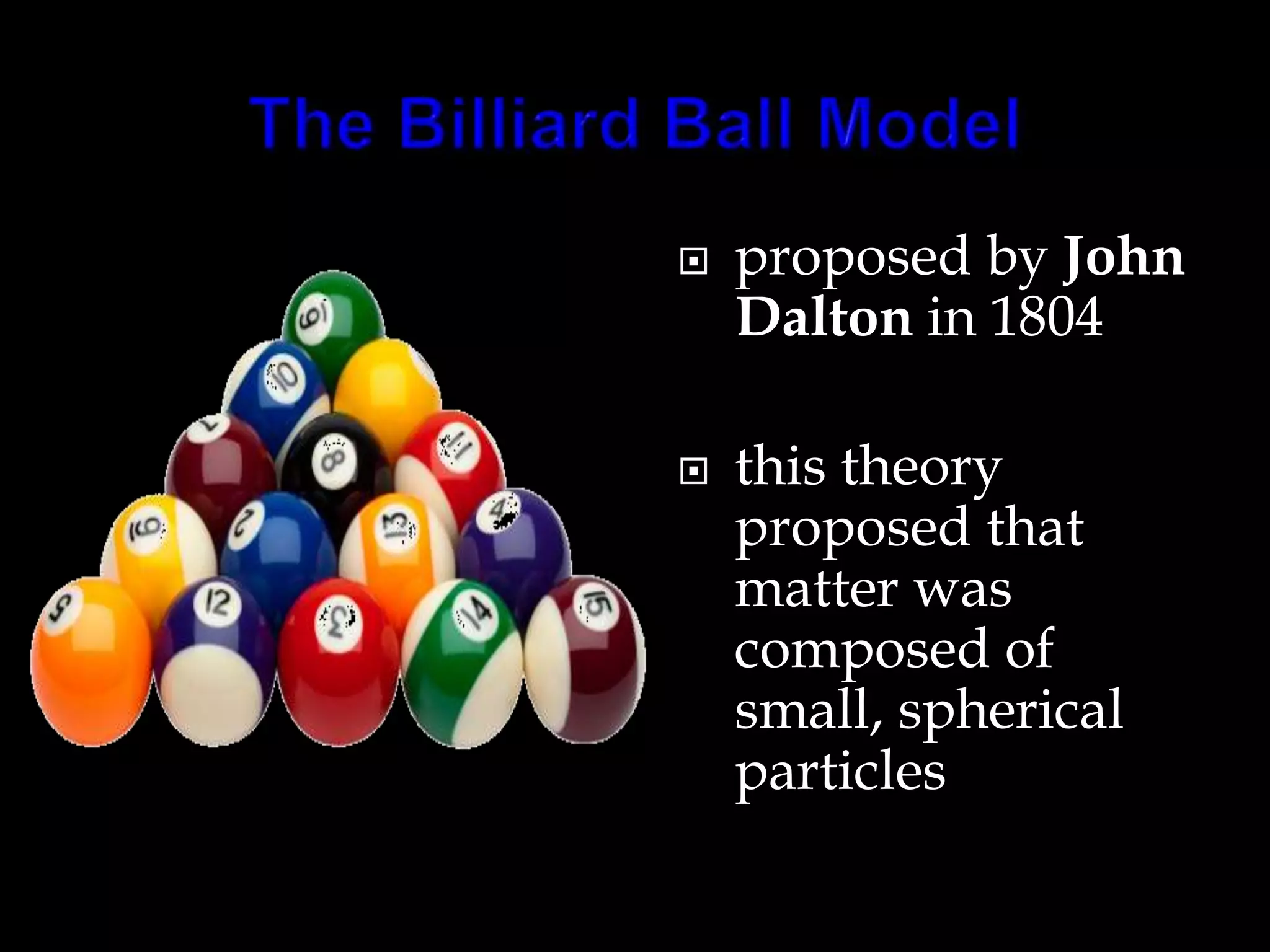
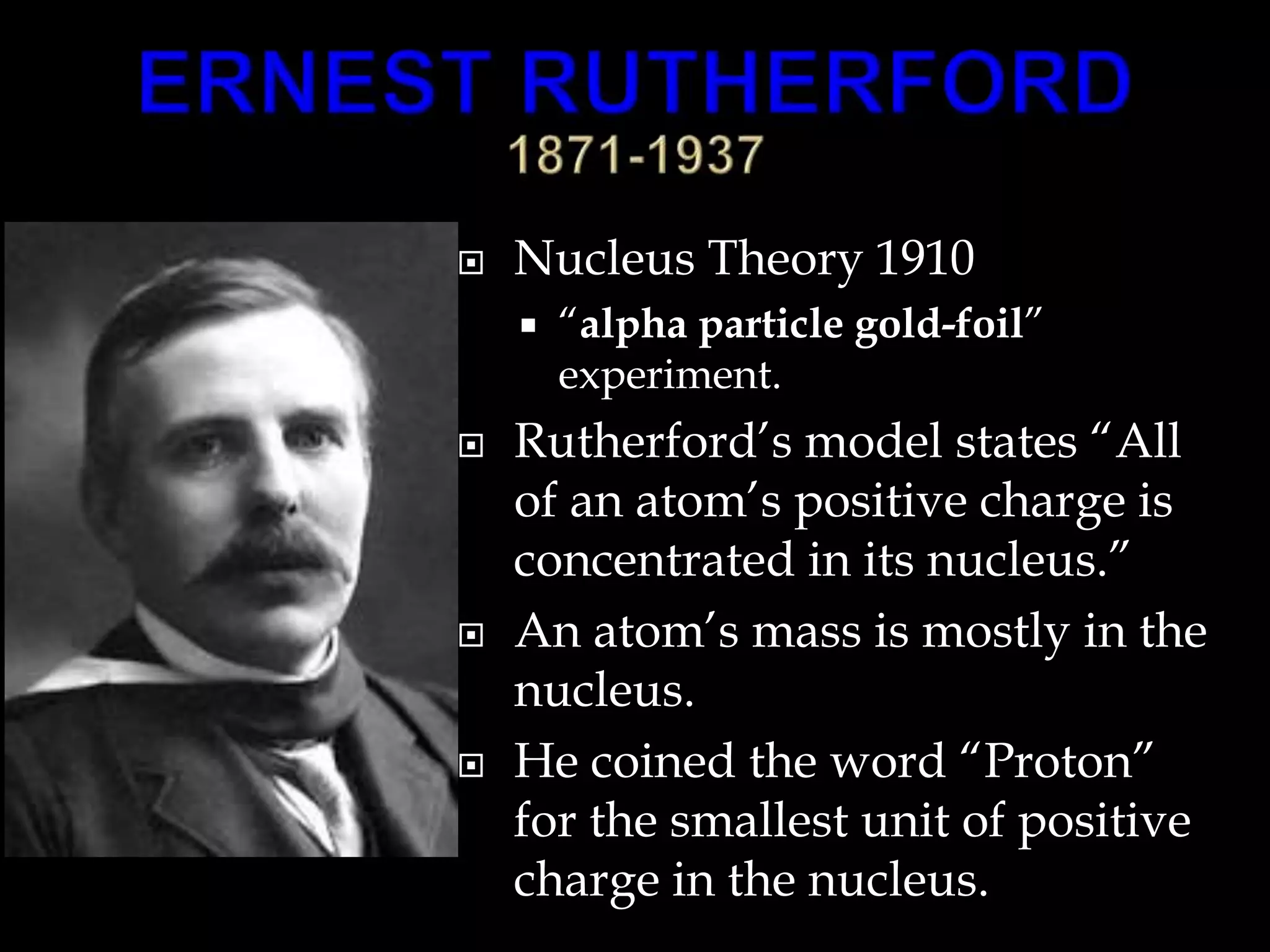
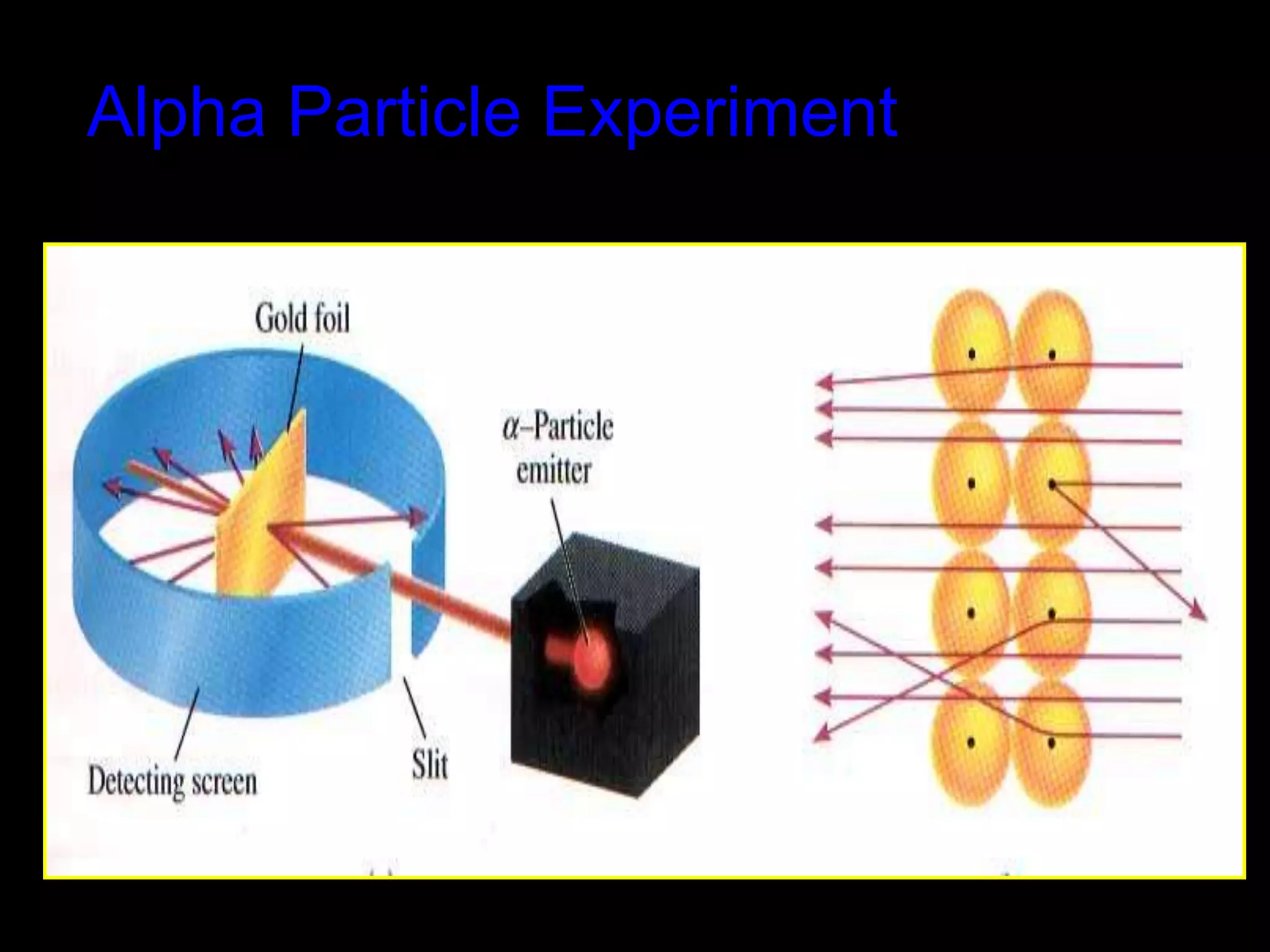
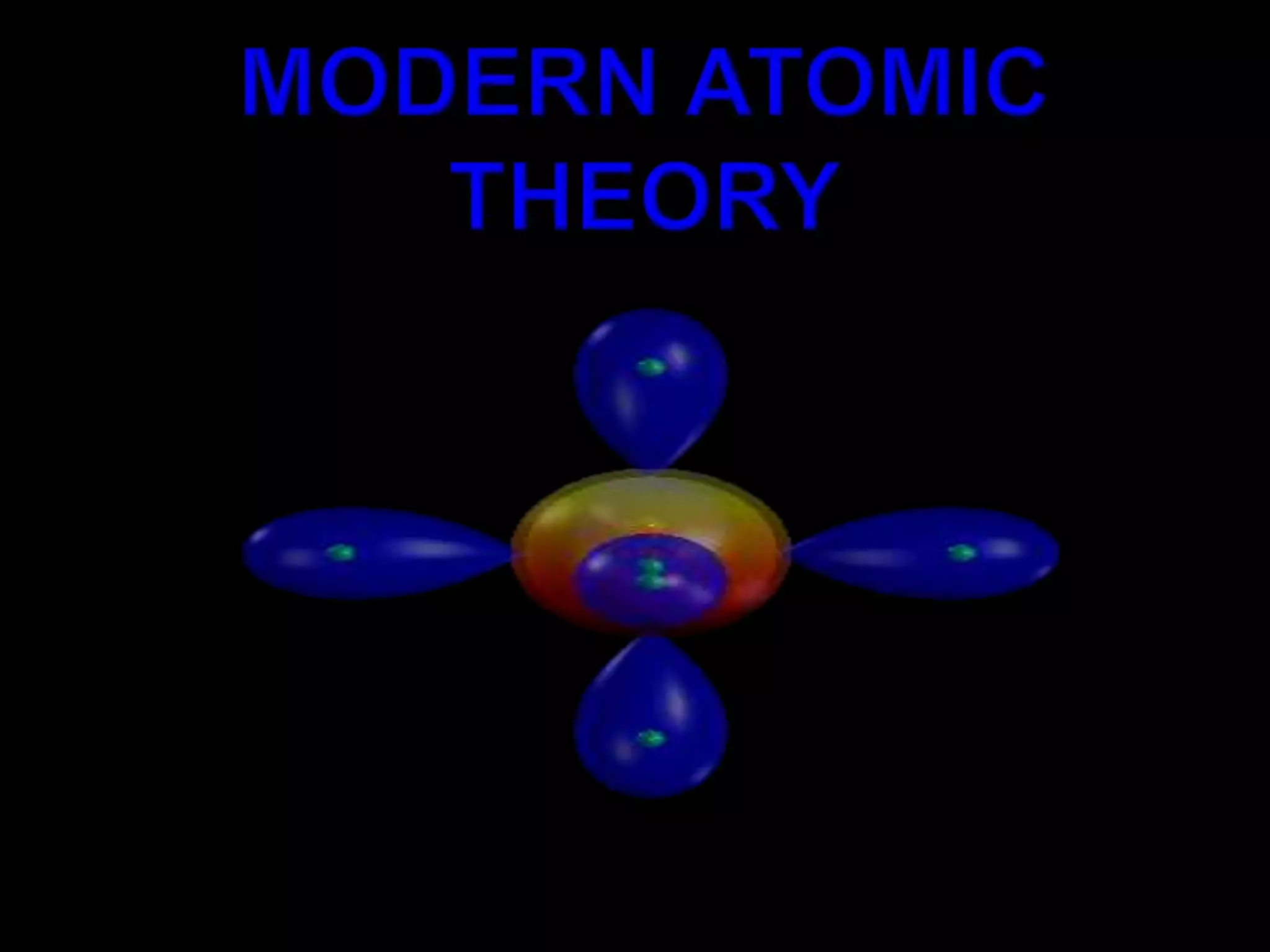
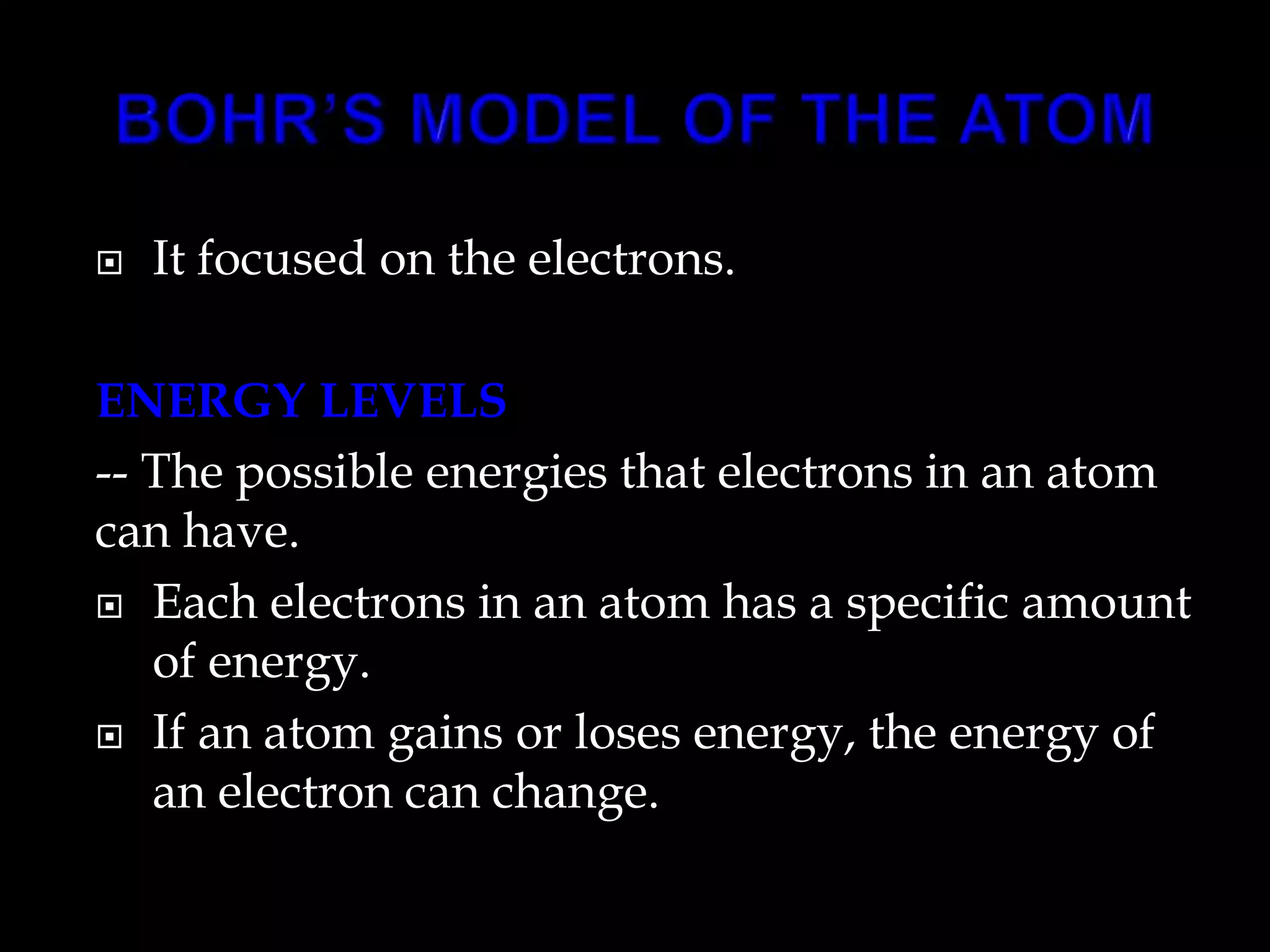
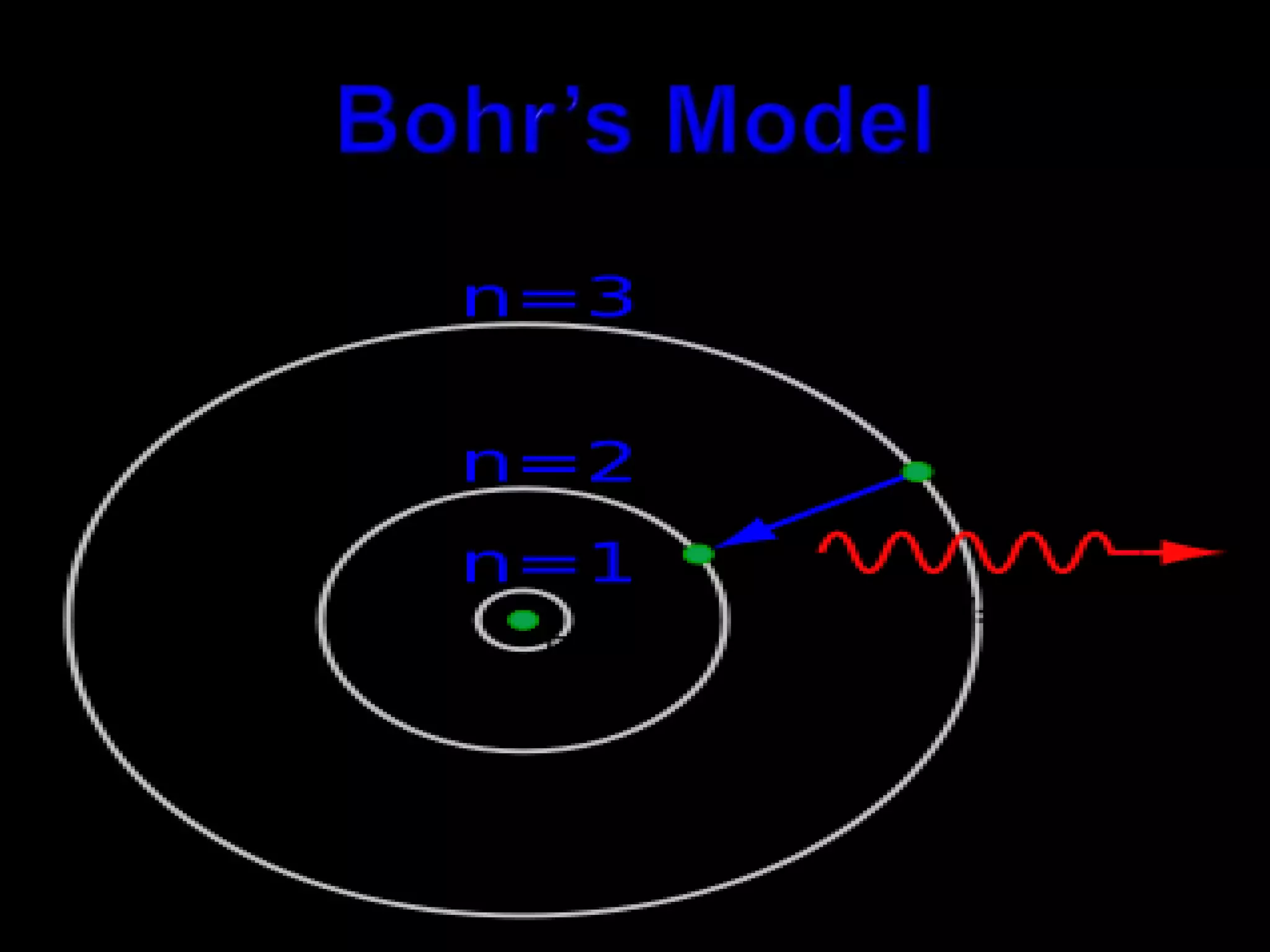
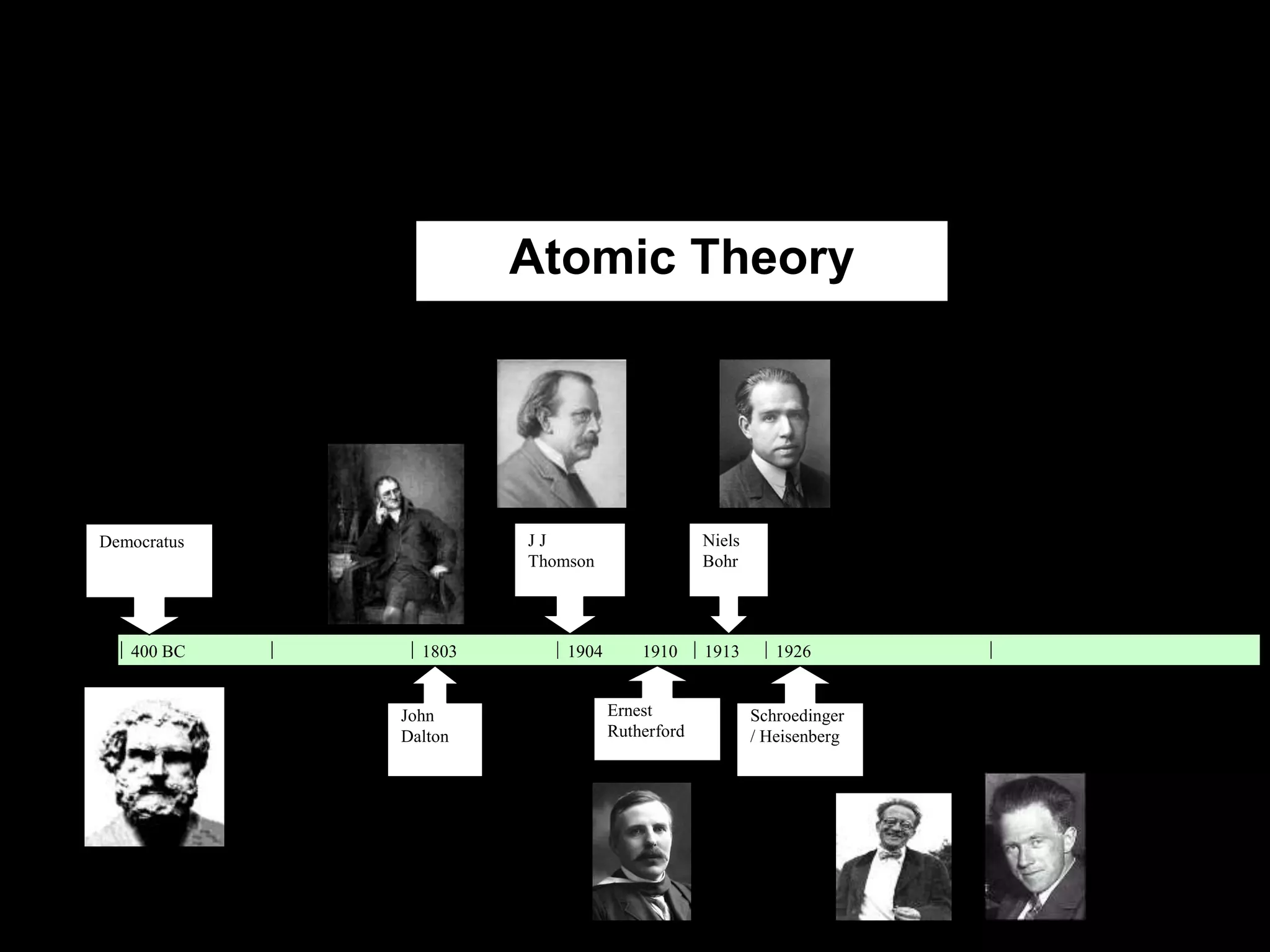
John Dalton proposed the atomic theory in 1804, stating that all matter is composed of tiny, indivisible particles called atoms that cannot be divided further. Later discoveries found that atoms consist of even smaller subatomic particles, including electrons discovered by J.J. Thomson in 1897 and the nucleus discovered by Ernest Rutherford in 1910. The quantum mechanical model developed in 1926 by Schrodinger, Heisenberg and others proposed that electrons exist as waves of energy around the nucleus, rather than following fixed orbits as proposed by Niels Bohr's 1913 planetary model of the atom.