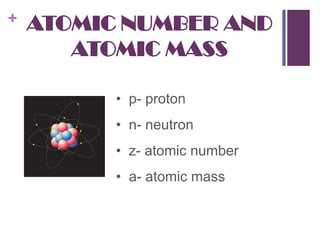
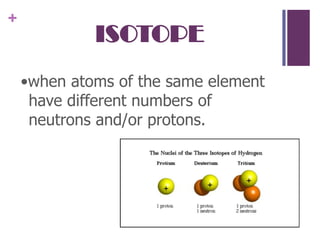
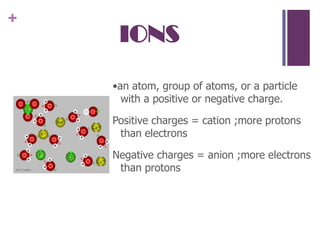
1. Democritus was the first to propose that all matter is composed of tiny indivisible units called atoms. Later scientists like Dalton expanded on this idea.
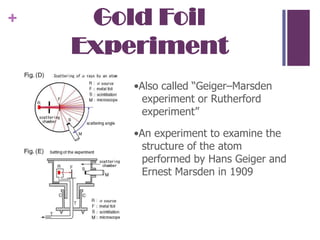
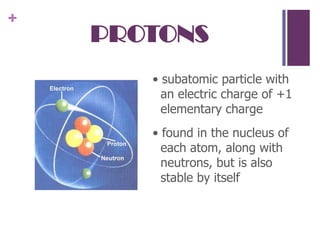
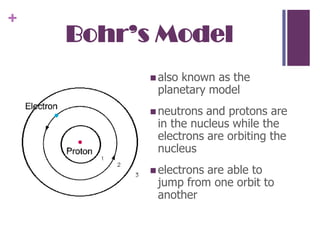
2. Rutherford's gold foil experiment showed that atoms have a small, dense nucleus containing protons and neutrons, with electrons orbiting the nucleus. This overturned the plum pudding model.
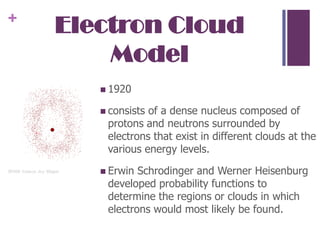
3. Chadwick discovered the neutron in 1932, completing the standard picture of atomic structure with protons and neutrons in the nucleus and electrons in orbitals around it.