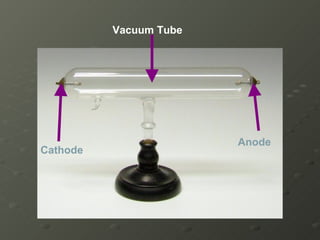
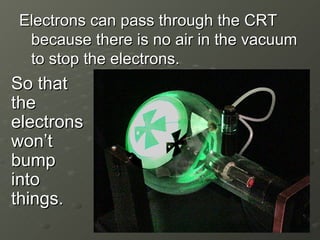
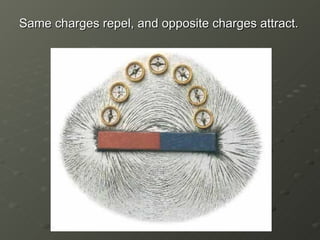
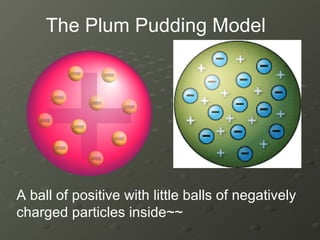
J.J. Thomson discovered electrons in 1897 using a cathode ray tube, which showed that cathode rays were streams of electrons. He proposed the plum pudding model of the atom, which depicted the atom as a ball of positive charge with negative electrons embedded inside. This contradicted Dalton's model of individual solid spheres, leading Thomson to disprove Dalton's model of atomic structure.