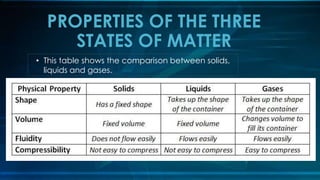
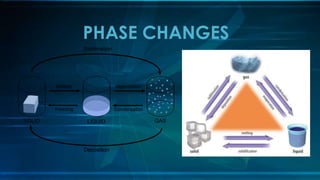
This document discusses the atomic theory and properties of the three states of matter. It covers key scientists and their contributions, including Dalton formulating the atomic theory, Thomson discovering the electron, Rutherford naming the types of radiation, and Bohr proposing electron orbitals. The three states of matter - solid, liquid, gas - are compared in terms of particle motion and forces. Phase changes like melting, freezing and evaporation are explained. The development of the modern atomic model and discovery of subatomic particles like the proton and neutron are also summarized.