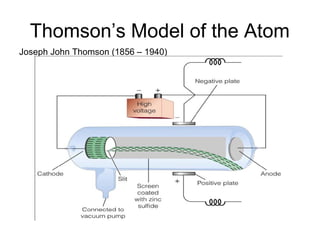
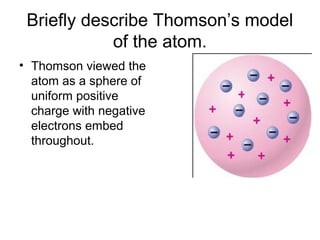
The document summarizes the development of atomic theory from ancient Greek philosophers to modern quantum mechanics. It describes early atomic models including Dalton's theory that atoms are indivisible particles that combine in whole number ratios, as well as Thomson's plum pudding model and Rutherford's nuclear model developed from his gold foil experiment. Later, Bohr incorporated quantized energy levels to explain atomic spectra, while quantum mechanics describes electrons as probabilistic clouds within orbitals and energy levels.