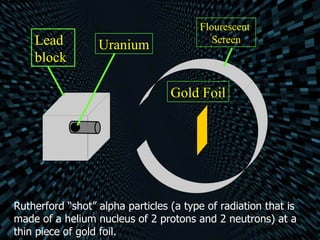
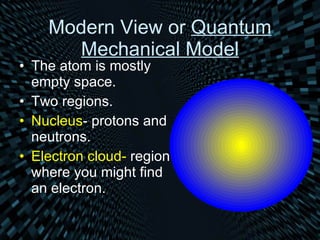
The document traces the history and development of atomic models from ancient Greek philosophers to modern quantum theory. It describes Democritus's early idea of indivisible atoms, followed by John Dalton formalizing the first atomic theory in the early 1800s. J.J. Thomson later discovered the electron and proposed that atoms are like "plum puddings" with positive matter and embedded electrons. Ernest Rutherford's gold foil experiment revealed that atoms have a small, dense positive nucleus, leading Niels Bohr to model electrons orbiting the nucleus in fixed shells. Modern quantum theory describes electrons as occupying probability clouds or orbitals around the nucleus.