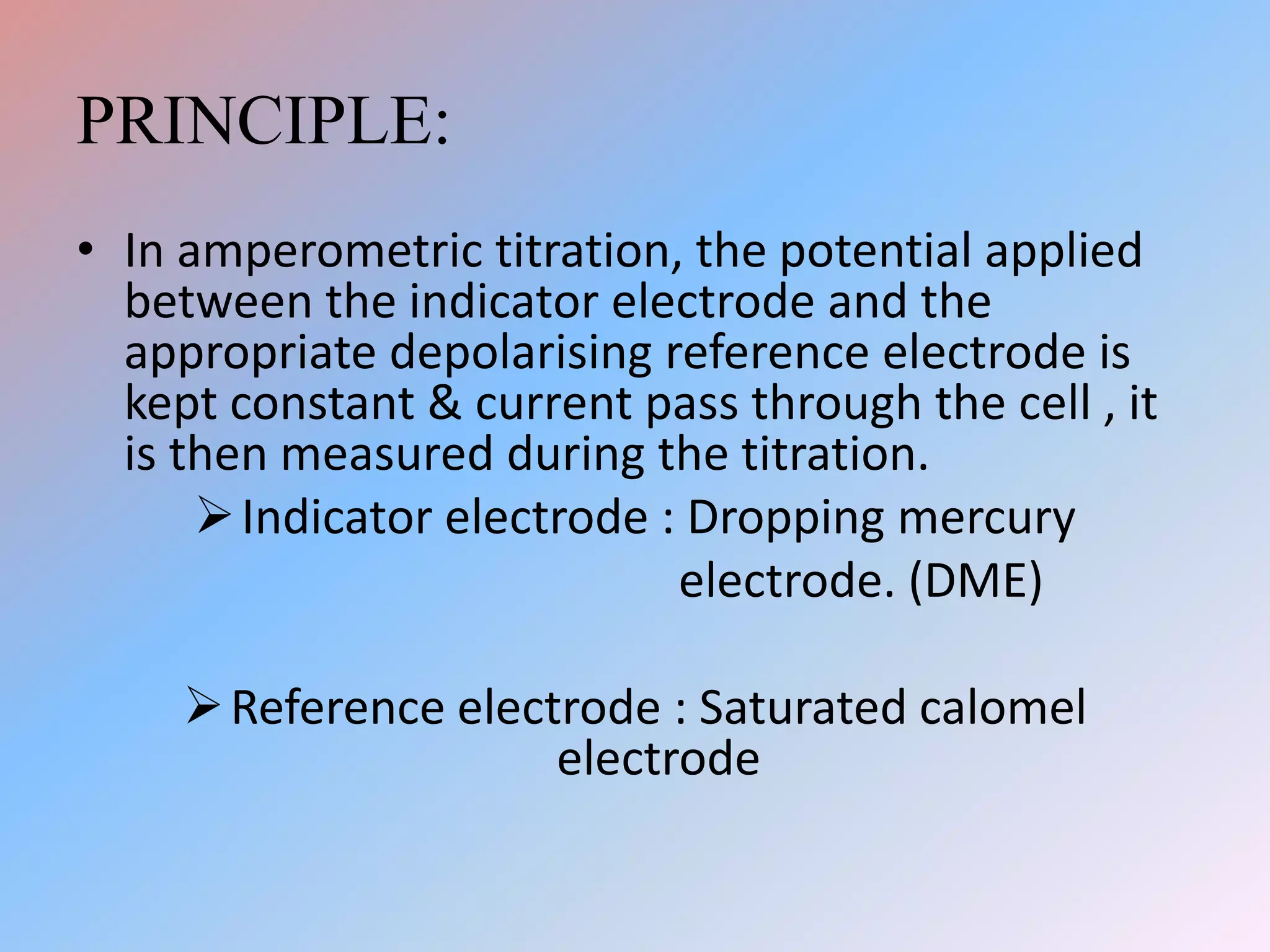
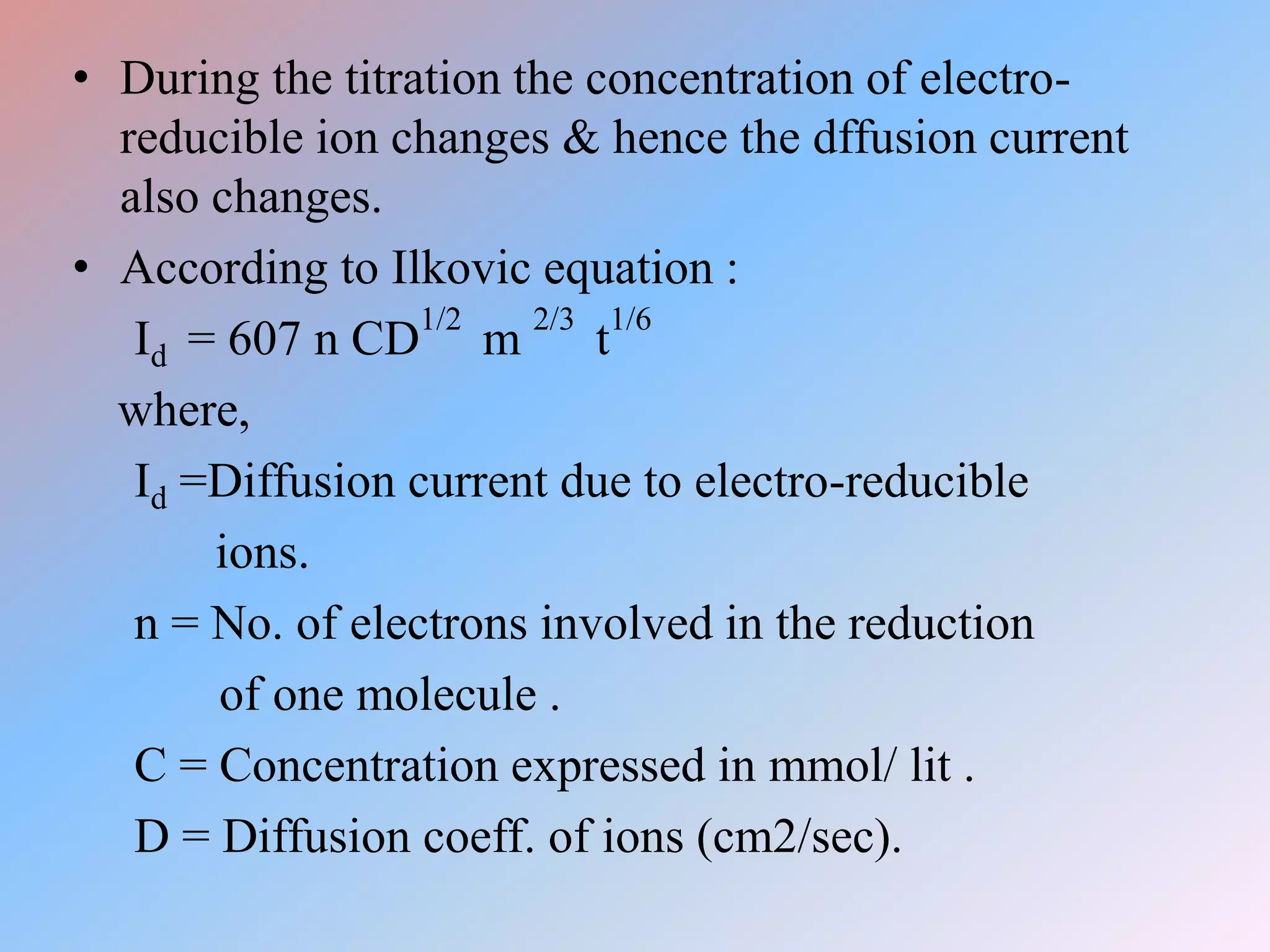
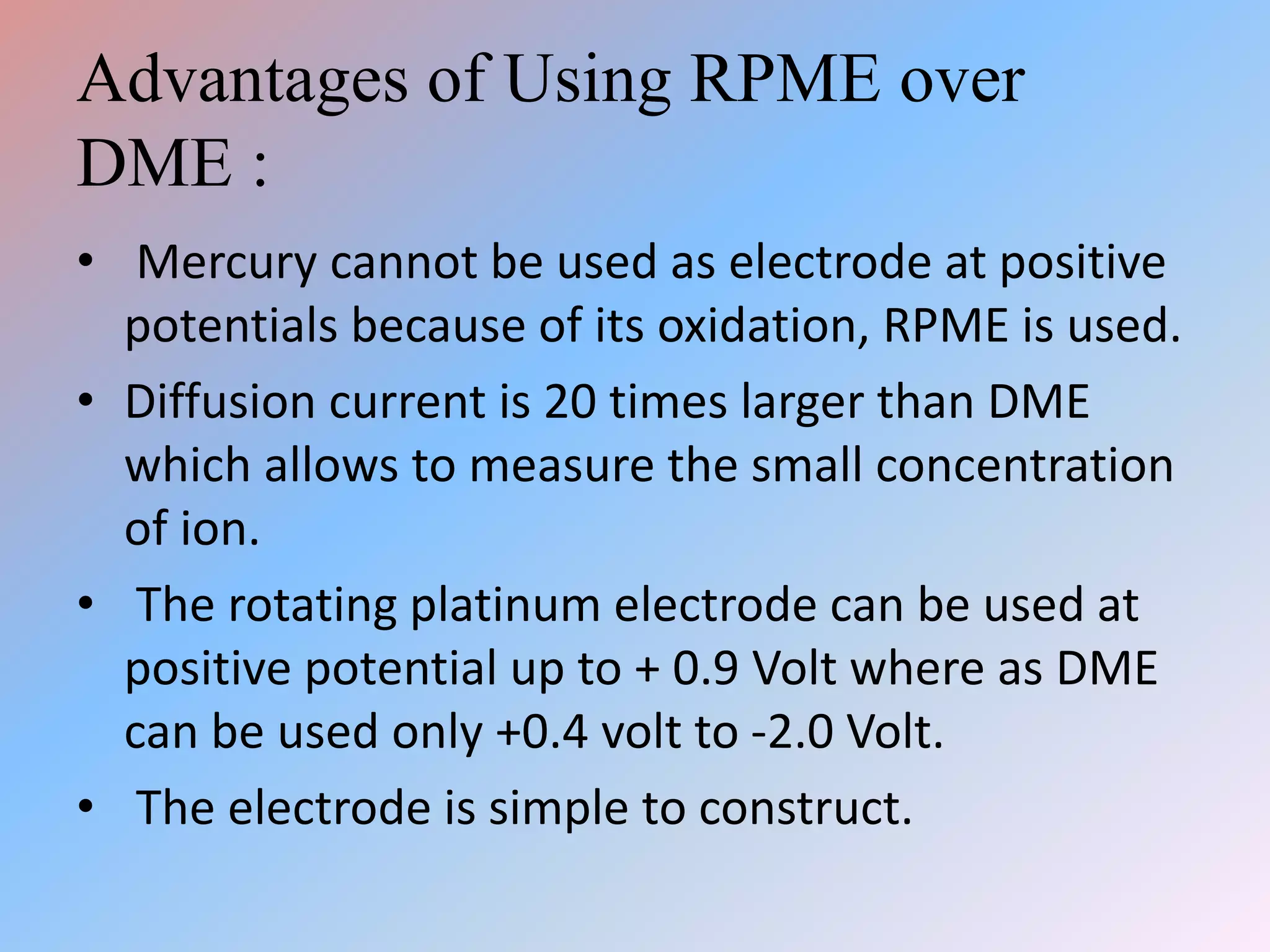
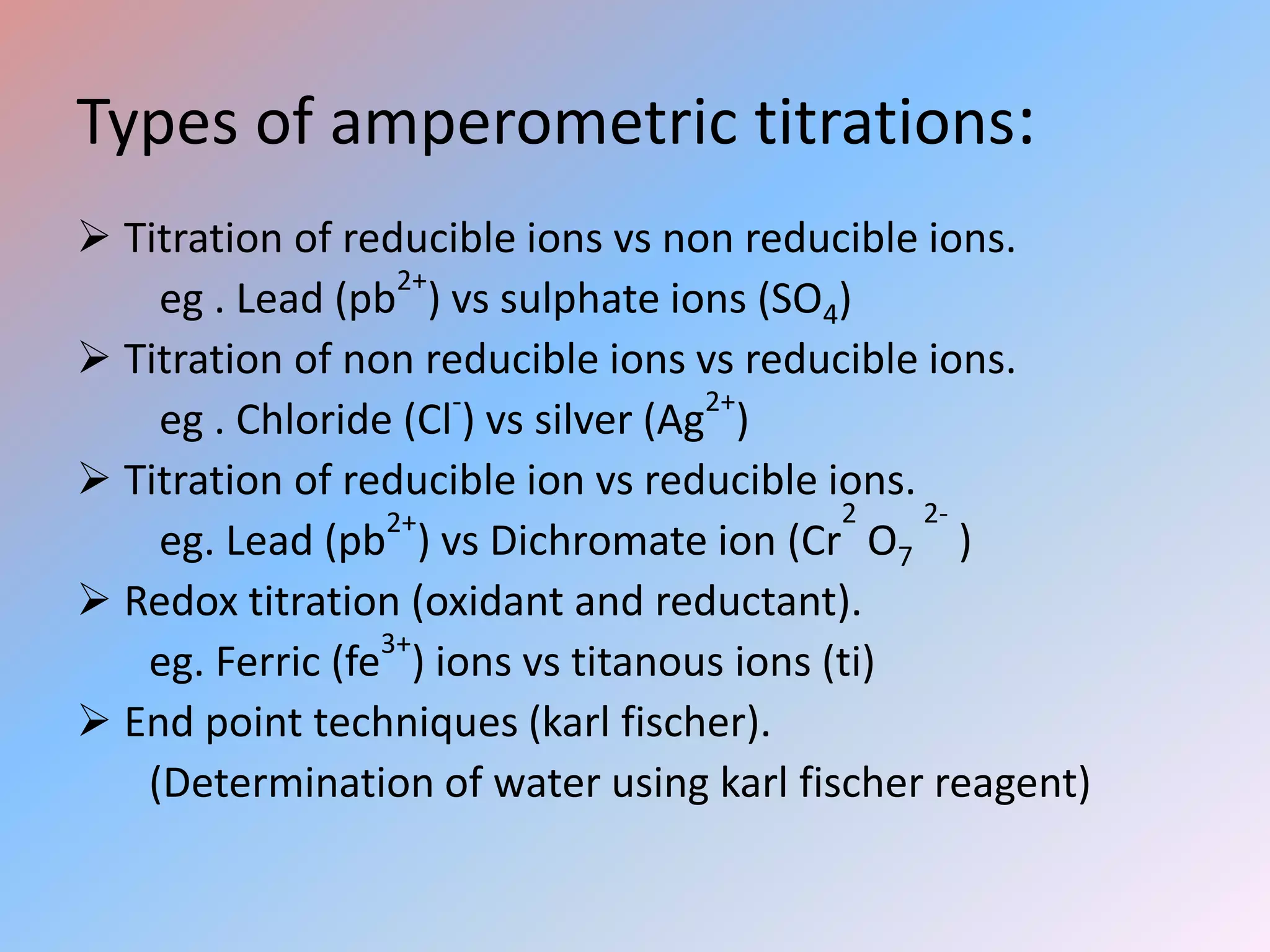
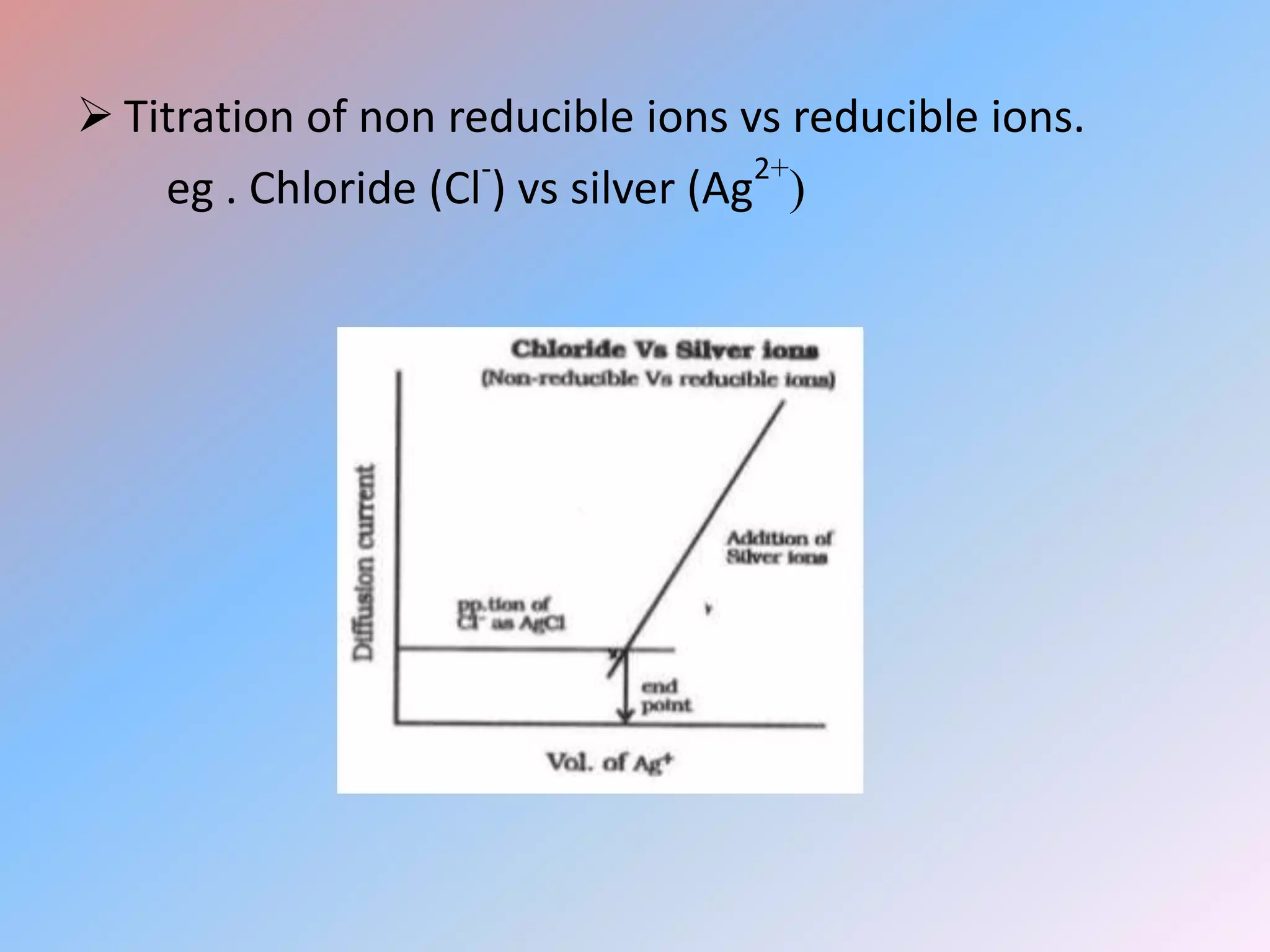
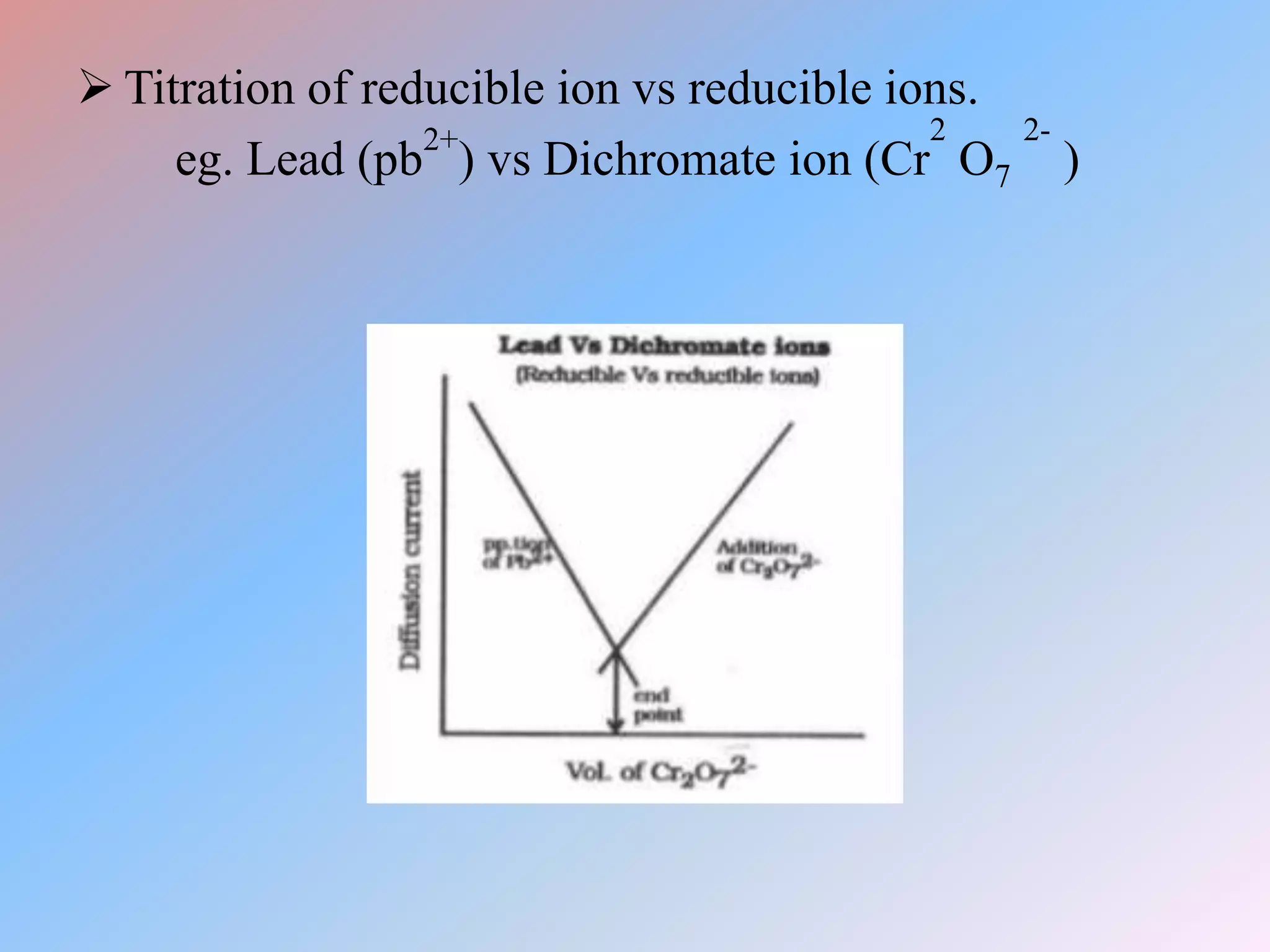
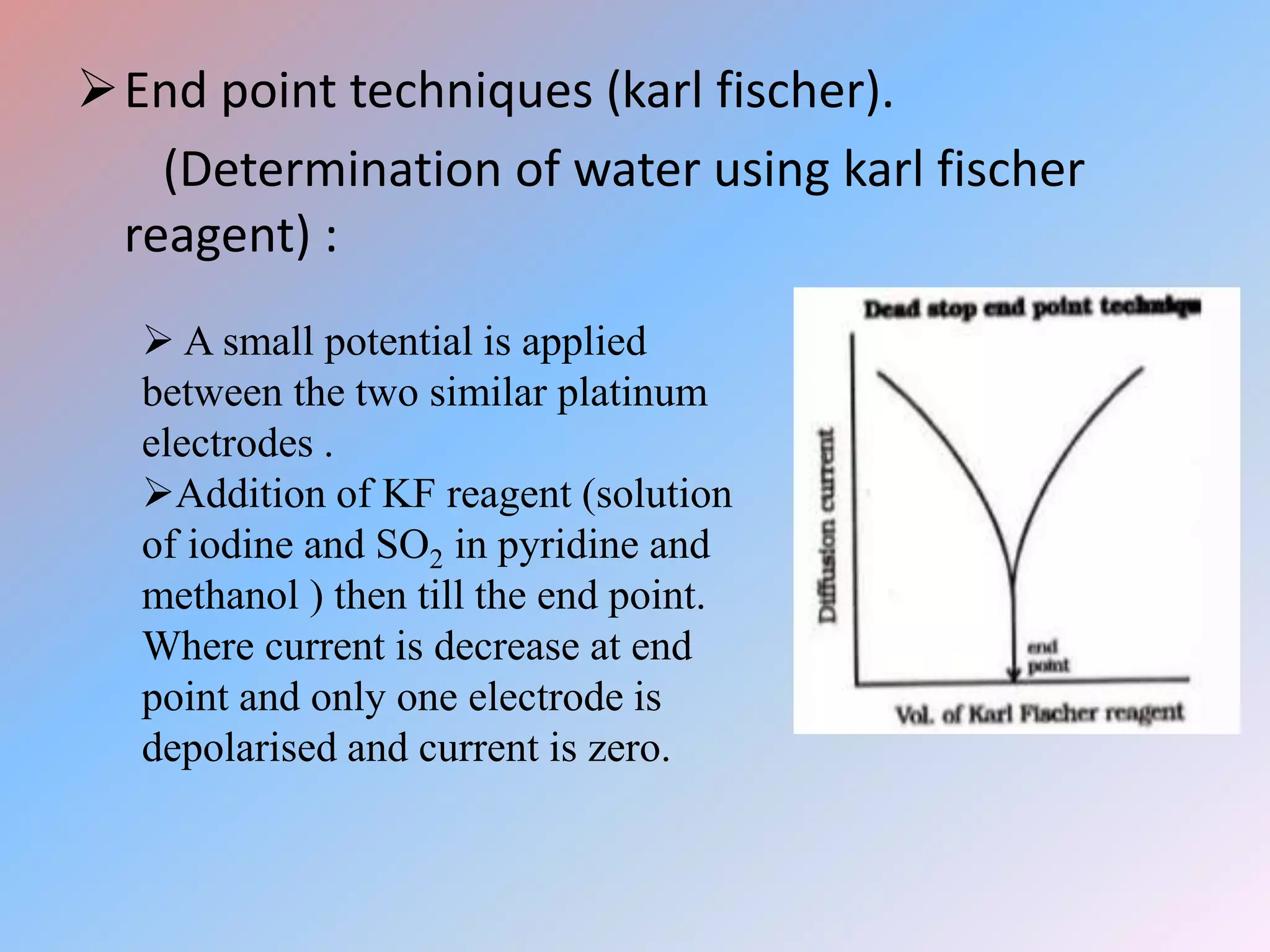
This document discusses amperometric titration, which is an electrochemical titration method that measures current under a constant applied voltage. It explains the principle that the current passing through an indicator electrode is measured during titration as the concentration of electroreducible ions changes. The document outlines the conditions, apparatus used including dropping mercury and rotating platinum microelectrodes, types of amperometric titrations, advantages such as ability to analyze reducible and non-reducible ions, applications including HPLC detection, and disadvantages like inaccurate results from foreign substances.