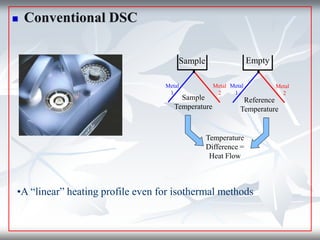
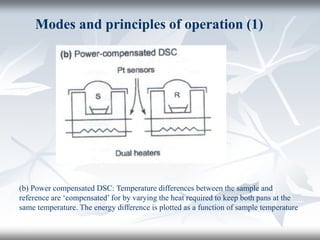
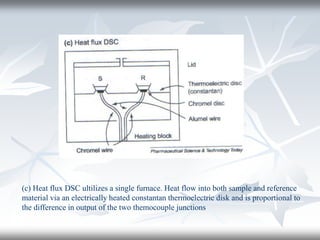
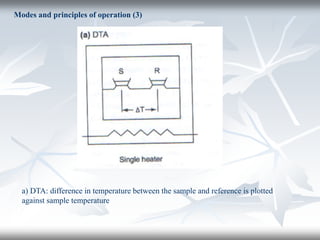
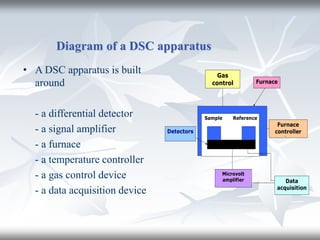
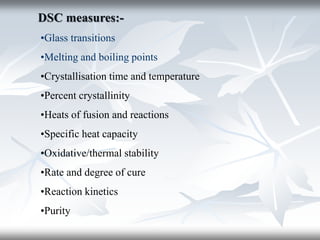
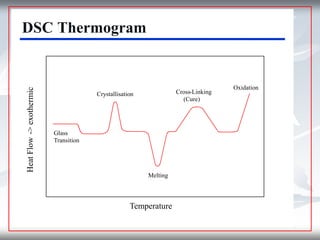
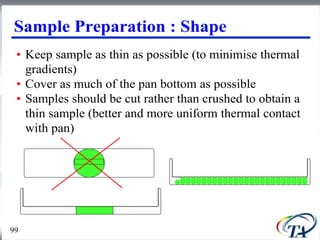
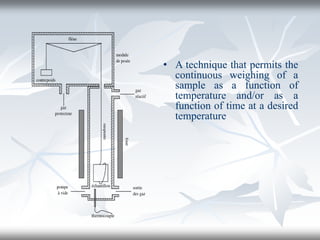
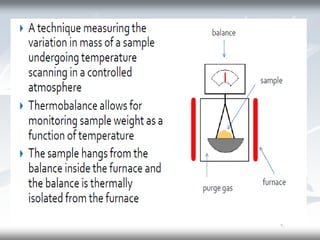
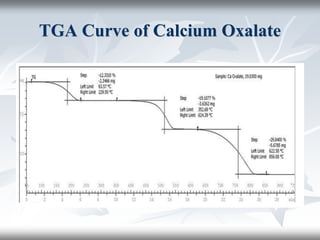
This document discusses Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC). TGA measures the change in weight of a sample during heating or cooling, while DSC measures the heat absorbed or released by a sample during phase transitions or chemical reactions. Both techniques provide information about physical and chemical changes in materials as functions of temperature. The document describes the principles, instrumentation, experimental procedures, sources of error, and applications of TGA and DSC for characterizing materials.