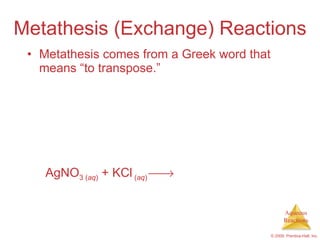This document summarizes key concepts in solution chemistry and stoichiometry, including:
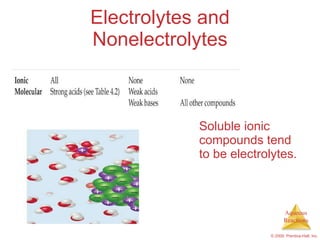
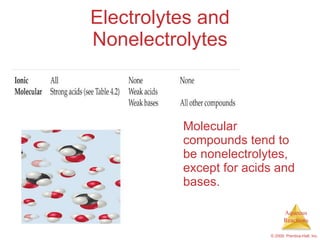
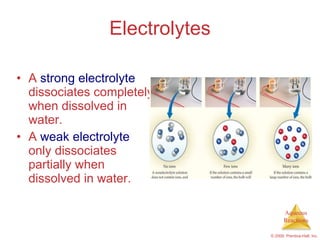
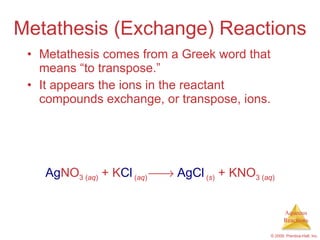
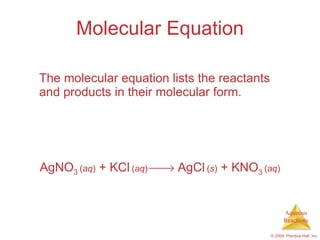
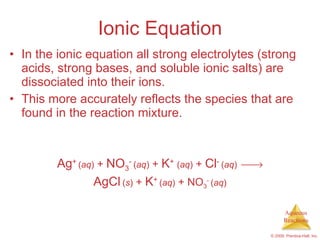
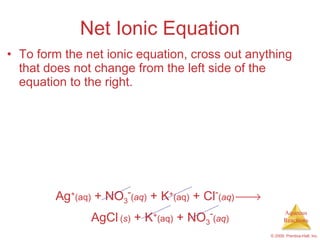
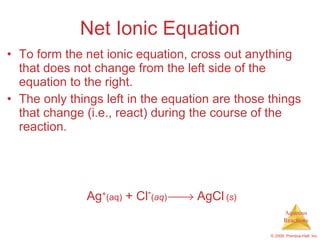
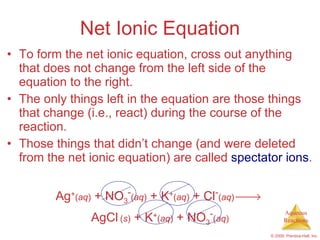
1) Solutions, electrolytes, dissociation, and precipitation reactions are discussed. Strong and weak electrolytes are defined.
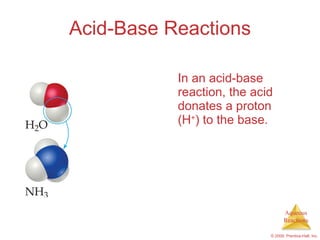
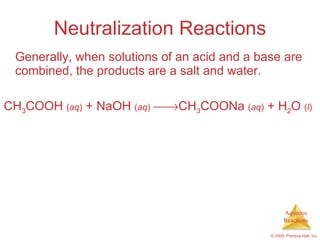
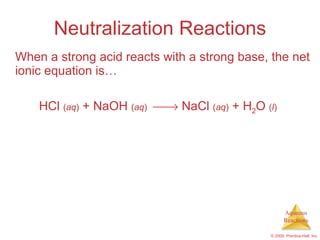
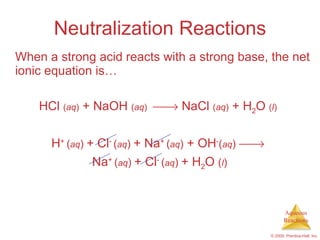
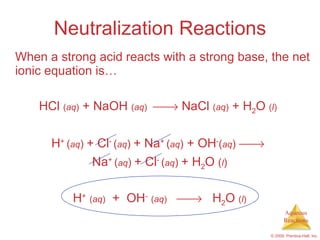
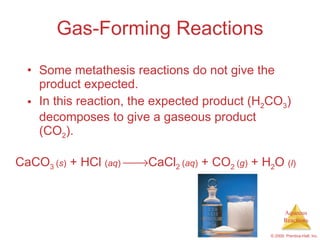
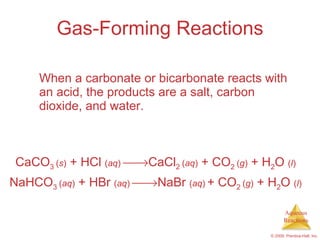
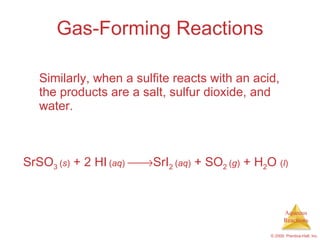
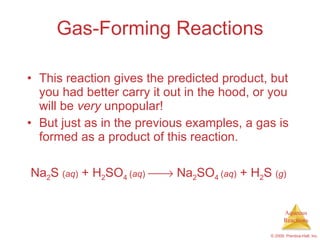
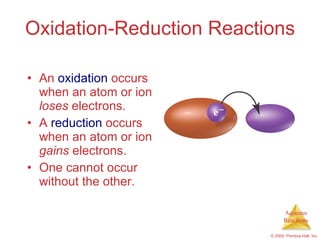
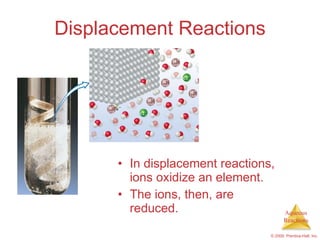
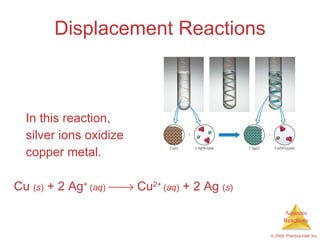
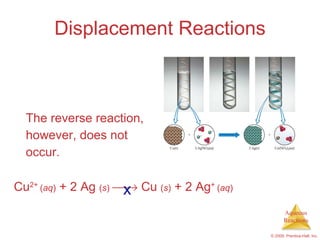
2) Acid-base reactions such as neutralization and gas-forming reactions are covered. Oxidation-reduction reactions and oxidation numbers are also introduced.
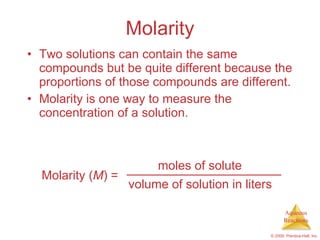
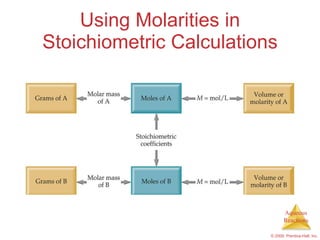
3) Concepts like molarity, dilution, and titration are explained as methods to quantify concentrations in solutions and chemical reactions.