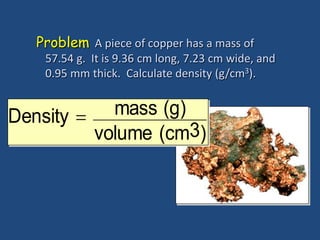
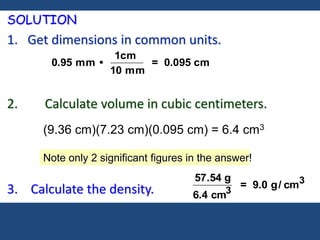
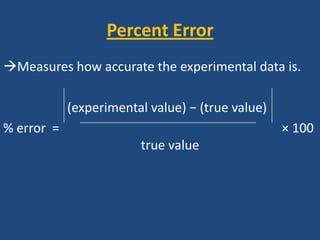
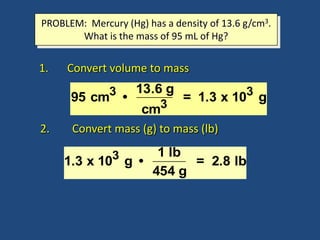
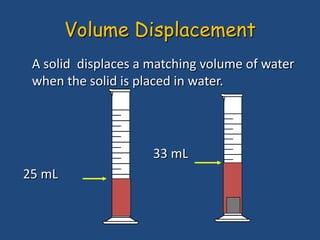
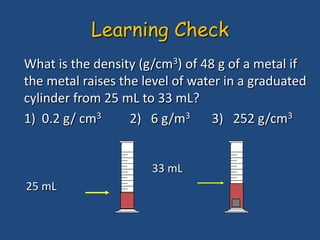
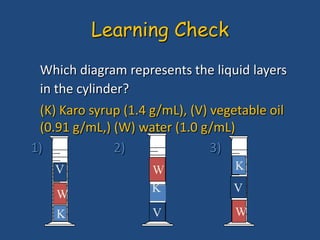
This document discusses density and provides examples of calculating density from mass and volume measurements. It defines density as an intensive property that does not depend on amount of matter. Density is the mass per unit volume and has consistent units of g/mL or g/cm3. Examples show calculating density of metals from given mass and volume, and calculating volume or mass when the other property and density are known. Percent error in density measurements is also explained.