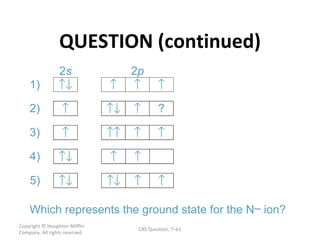
The document contains questions and answers related to chemistry concepts covered in chapters 6 and 7, including:
1. Thermodynamics concepts such as internal energy, enthalpy, and Hess's Law.
2. Properties of light and electromagnetic radiation including wavelength, frequency, and photon energy.
3. Models of the atom including the Bohr model and quantum mechanical model.
4. Electronic structure of atoms including orbitals, quantum numbers, and periodic trends.
























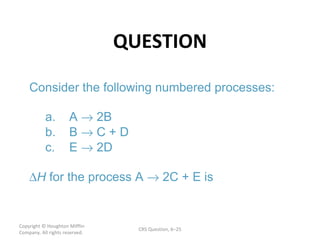
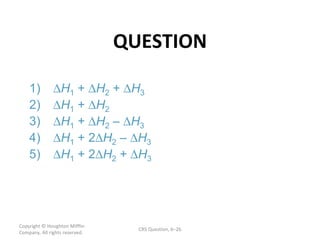














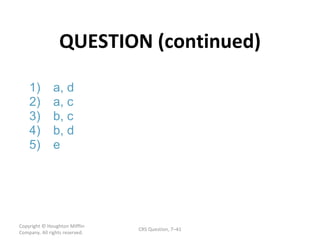












![ANSWER Copyright © Houghton Mifflin Company. All rights reserved. CRS Question, 7 – 2 - 2) [Xe] 6 s Section 7.11 The Aufbau Principle and the Periodic Table (p. 302) [Xe] denotes a shorthand version of the electron configuration for Xe. Noble gas configurations are used to reduce writing time.](https://image.slidesharecdn.com/apchem-chapters6and7-091106194307-phpapp02/85/Ap-Chem-Chapters-6-And-7-54-320.jpg)