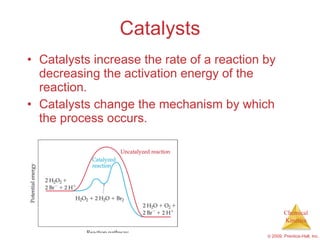
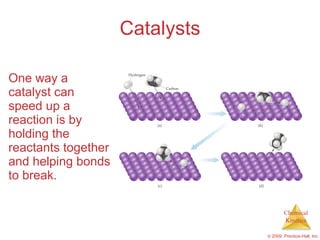
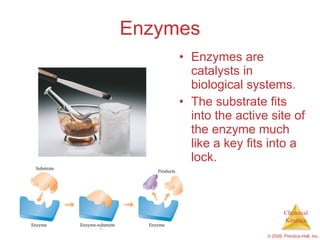
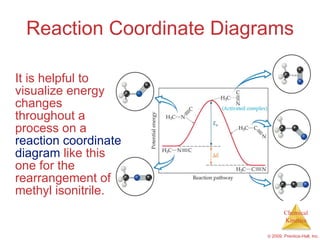
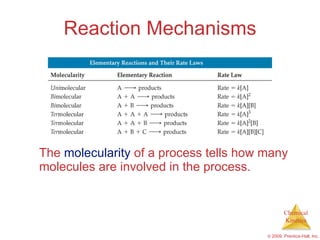
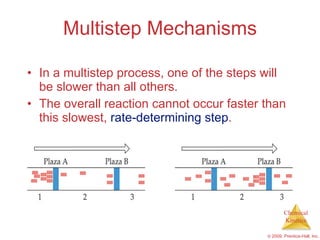
This document discusses chemical kinetics and reaction rates. It explains that kinetics studies how fast chemical reactions occur. The rate of a reaction depends on factors like the concentrations of reactants, temperature, and presence of catalysts. Reaction rates can be determined by measuring changes in concentration over time. The order of a reaction indicates how the rate depends on reactant concentrations. First-order and second-order reactions follow distinct rate laws that allow calculation of rate constants from experimental data. Reaction mechanisms involve elementary steps that describe the pathway by which reactants are converted to products.






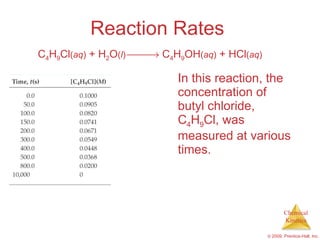
![Reaction Rates The average rate of the reaction over each interval is the change in concentration divided by the change in time: C 4 H 9 Cl ( aq ) + H 2 O ( l ) C 4 H 9 OH ( aq ) + HCl ( aq ) Average rate = [C 4 H 9 Cl] t](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-9-320.jpg)
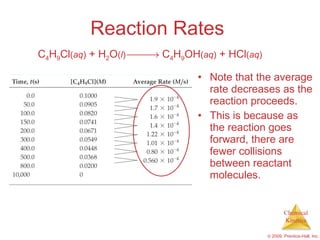
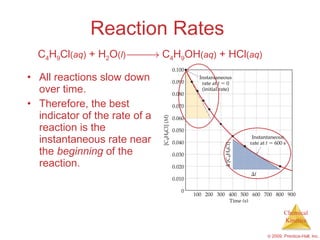
![Reaction Rates A plot of [C 4 H 9 Cl] vs. time for this reaction yields a curve like this. The slope of a line tangent to the curve at any point is the instantaneous rate at that time. C 4 H 9 Cl ( aq ) + H 2 O ( l ) C 4 H 9 OH ( aq ) + HCl ( aq )](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-11-320.jpg)
![Reaction Rates and Stoichiometry In this reaction, the ratio of C 4 H 9 Cl to C 4 H 9 OH is 1:1. Thus, the rate of disappearance of C 4 H 9 Cl is the same as the rate of appearance of C 4 H 9 OH. C 4 H 9 Cl ( aq ) + H 2 O ( l ) C 4 H 9 OH ( aq ) + HCl ( aq ) Rate = - [C 4 H 9 Cl] t = [C 4 H 9 OH] t](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-13-320.jpg)
![Reaction Rates and Stoichiometry What if the ratio is not 1:1? 2 HI ( g ) H 2 ( g ) + I 2 ( g ) In such a case , Rate = − 1 2 [HI] t = [I 2 ] t](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-14-320.jpg)
![Reaction Rates and Stoichiometry To generalize, then, for the reaction a A + b B c C + d D Rate = − 1 a [A] t = − 1 b [B] t = 1 c [C] t 1 d [D] t =](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-15-320.jpg)

![Concentration and Rate If we compare Experiments 1 and 2, we see that when [NH 4 + ] doubles, the initial rate doubles. NH 4 + ( aq ) + NO 2 − ( aq ) N 2 ( g ) + 2 H 2 O ( l )](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-17-320.jpg)
![Concentration and Rate Likewise, when we compare Experiments 5 and 6, we see that when [NO 2 − ] doubles, the initial rate doubles. NH 4 + ( aq ) + NO 2 − ( aq ) N 2 ( g ) + 2 H 2 O ( l )](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-18-320.jpg)
![Concentration and Rate This means Rate [NH 4 + ] Rate [NO 2 − ] Rate [NH 4 + ] [NO 2 − ] which, when written as an equation, becomes Rate = k [NH 4 + ] [NO 2 − ] This equation is called the rate law , and k is the rate constant . Therefore,](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-19-320.jpg)
![Rate Laws A rate law shows the relationship between the reaction rate and the concentrations of reactants. The exponents tell the order of the reaction with respect to each reactant. Since the rate law is Rate = k [NH 4 + ] [NO 2 − ] the reaction is First-order in [NH 4 + ] and First-order in [NO 2 − ].](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-20-320.jpg)
![Rate Laws Rate = k [NH 4 + ] [NO 2 − ] The overall reaction order can be found by adding the exponents on the reactants in the rate law. This reaction is second-order overall.](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-21-320.jpg)
![Integrated Rate Laws Using calculus to integrate the rate law for a first-order process gives us Where [A] 0 is the initial concentration of A, and [A] t is the concentration of A at some time, t , during the course of the reaction. ln [A] t [A] 0 = − k t](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-22-320.jpg)
![Integrated Rate Laws Manipulating this equation produces… ln [A] t − ln [A] 0 = − k t ln [A] t = − k t + ln [A] 0 … which is in the form y = mx + b ln [A] t [A] 0 = − k t](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-23-320.jpg)
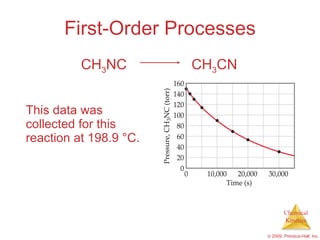
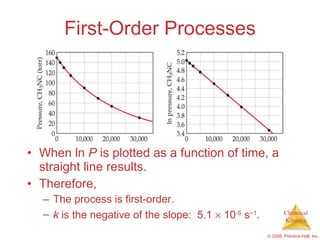
![First-Order Processes Therefore, if a reaction is first-order, a plot of ln [A] vs. t will yield a straight line, and the slope of the line will be - k . ln [A] t = - k t + ln [A] 0](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-24-320.jpg)
![Second-Order Processes Similarly, integrating the rate law for a process that is second-order in reactant A, we get also in the form y = mx + b 1 [A] t = kt + 1 [A] 0](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-28-320.jpg)
![Second-Order Processes So if a process is second-order in A, a plot of vs. t will yield a straight line, and the slope of that line is k . 1 [A] t = kt + 1 [A] 0 1 [A]](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-29-320.jpg)
![Second-Order Processes The decomposition of NO 2 at 300 ° C is described by the equation and yields data comparable to this: NO 2 ( g ) NO ( g ) + O 2 ( g ) 0.00380 300.0 0.00481 200.0 0.00649 100.0 0.00787 50.0 0.01000 0.0 [NO 2 ], M Time ( s ) 1 2](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-30-320.jpg)
![Second-Order Processes Plotting ln [NO 2 ] vs . t yields the graph at the right. The plot is not a straight line, so the process is not first-order in [A]. 0.00380 0.00481 0.00649 0.00787 0.01000 [NO 2 ], M − 5.573 − 5.337 − 5.038 − 4.845 − 4.610 ln [NO 2 ] 300.0 200.0 100.0 50.0 0.0 Time ( s )](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-31-320.jpg)
![Second-Order Processes Graphing ln vs. t , however, gives this plot. Because this is a straight line, the process is second-order in [A]. 0.00380 0.00481 0.00649 0.00787 0.01000 [NO 2 ], M 263 208 154 127 100 1/[NO 2 ] 300.0 200.0 100.0 50.0 0.0 Time ( s ) 1 [NO 2 ]](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-32-320.jpg)
![Half-Life Half-life is defined as the time required for one-half of a reactant to react. Because [A] at t 1/2 is one-half of the original [A], [A] t = 0.5 [A] 0 .](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-33-320.jpg)
![Half-Life For a first-order process, this becomes ln 0.5 = − kt 1/2 − 0.693 = − kt 1/2 NOTE: For a first-order process, then, the half-life does not depend on [A] 0 . 0.5 [A] 0 [A] 0 ln = − kt 1/2 = t 1/2 0.693 k](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-34-320.jpg)
![Half-Life For a second-order process, 1 0.5 [A] 0 = kt 1/2 + 1 [A] 0 2 [A] 0 = kt 1/2 + 1 [A] 0 2 − 1 [A] 0 = kt 1/2 1 [A] 0 = = t 1/2 1 k [A] 0](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-35-320.jpg)



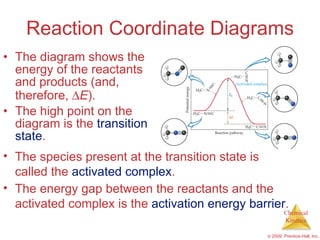
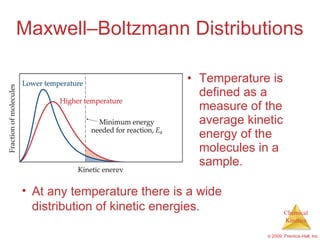
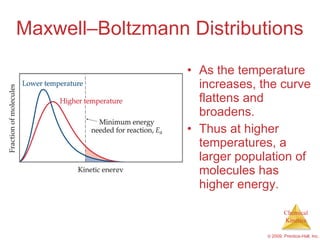
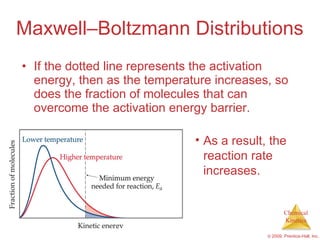

![Slow Initial Step The rate law for this reaction is found experimentally to be Rate = k [NO 2 ] 2 CO is necessary for this reaction to occur, but the rate of the reaction does not depend on its concentration. This suggests the reaction occurs in two steps. NO 2 ( g ) + CO ( g ) NO ( g ) + CO 2 ( g )](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-52-320.jpg)

![Fast Initial Step The rate law for this reaction is found to be Rate = k [NO] 2 [Br 2 ] Because termolecular processes are rare, this rate law suggests a two-step mechanism. 2 NO ( g ) + Br 2 ( g ) 2 NOBr ( g )](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-54-320.jpg)

![Fast Initial Step The rate of the overall reaction depends upon the rate of the slow step. The rate law for that step would be Rate = k 2 [NOBr 2 ] [NO] But how can we find [NOBr 2 ]?](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-56-320.jpg)

![Fast Initial Step Because Rate f = Rate r , k 1 [NO] [Br 2 ] = k − 1 [NOBr 2 ] Solving for [NOBr 2 ] gives us k 1 k − 1 [NO] [Br 2 ] = [NOBr 2 ]](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-58-320.jpg)
![Fast Initial Step Substituting this expression for [NOBr 2 ] in the rate law for the rate-determining step gives = k [NO] 2 [Br 2 ] k 2 k 1 k − 1 Rate = [NO] [Br 2 ] [NO]](https://image.slidesharecdn.com/ch14outline-100607173232-phpapp01/85/AP-Chemistry-Chapter-14-Outline-59-320.jpg)