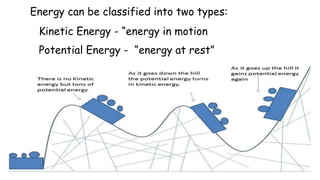
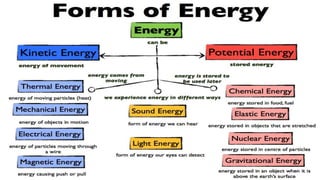
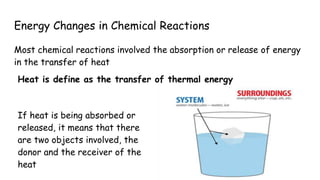
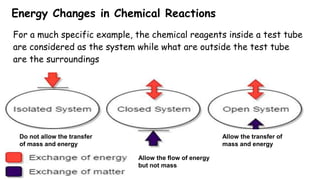
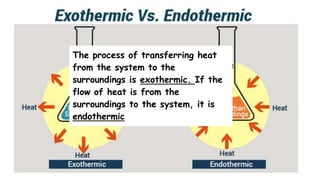
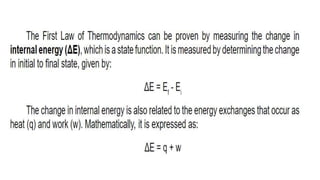
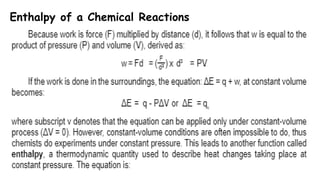
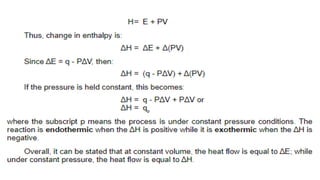
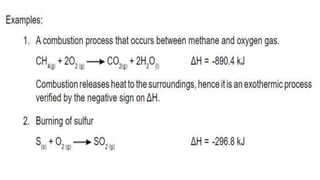
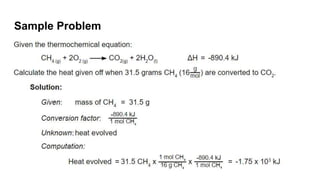
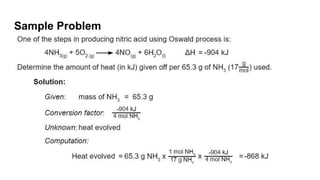
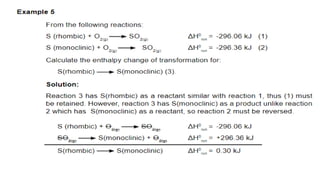
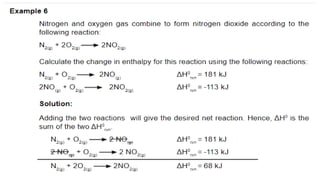
Thermochemistry is the study of heat energy associated with chemical reactions and physical transformations. A reaction may release or absorb energy during these processes. There are two types of energy: kinetic energy which is energy in motion, and potential energy which is energy at rest. The first law of thermodynamics states that energy is neither created nor destroyed, just changed from one form to another. Enthalpy (H) is a measurement of the total energy of a chemical reaction. Hess's law says the enthalpy change of a reaction is independent of the pathway taken and depends only on the initial and final states.