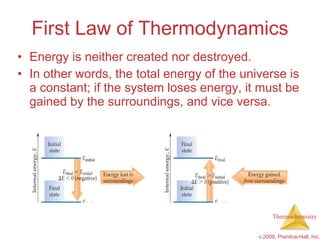
This document summarizes key concepts in thermochemistry, including:
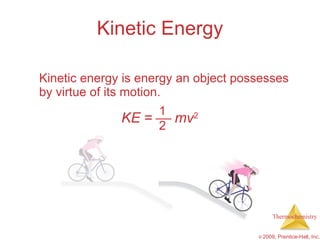
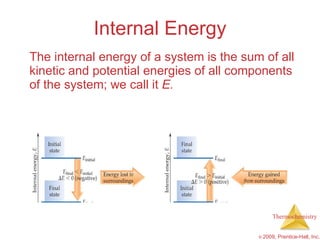
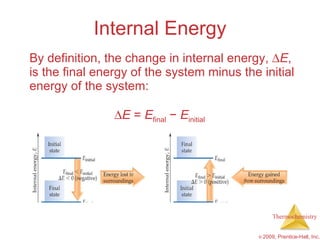
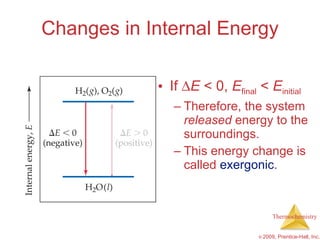
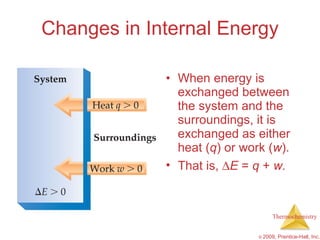
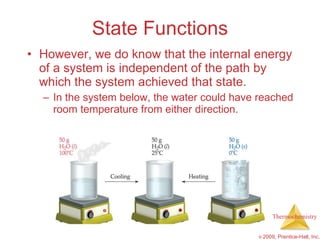
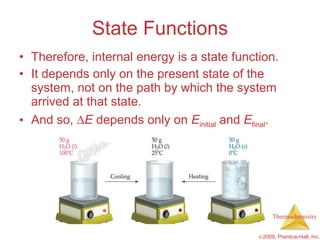
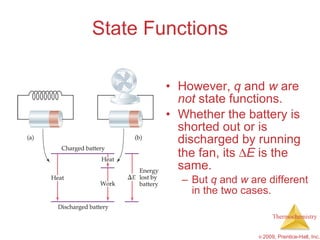
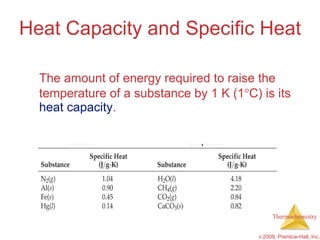
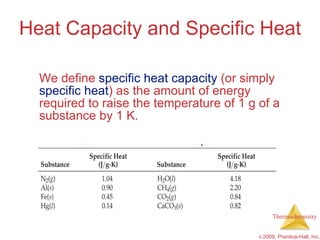
1) Energy can be transferred as heat or work. The SI unit of energy is the joule. Internal energy is the sum of kinetic and potential energies within a system.
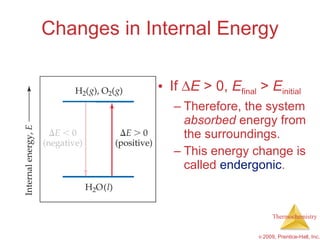
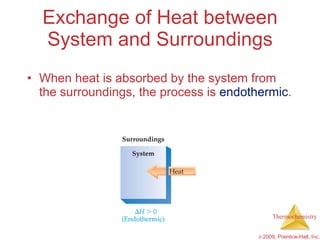
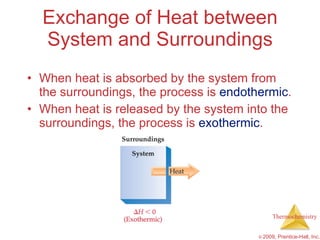
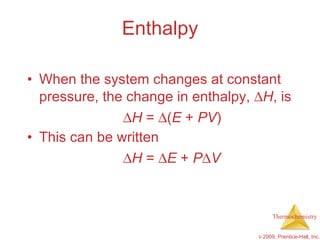
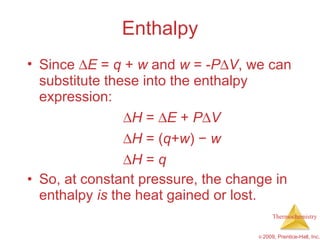
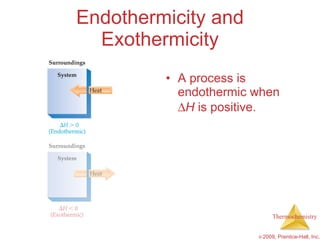
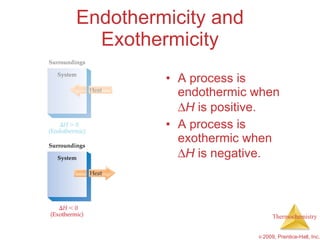
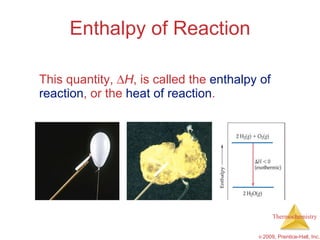
2) Enthalpy is a measure of the total energy of a system at constant pressure. It can be used to determine whether chemical reactions are endothermic or exothermic.
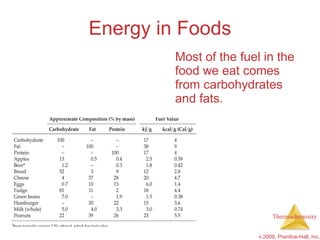
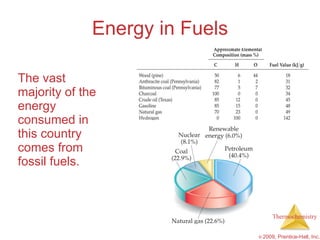
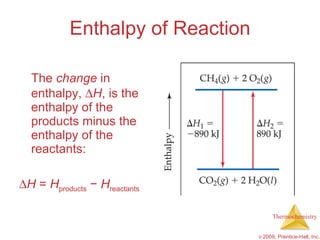
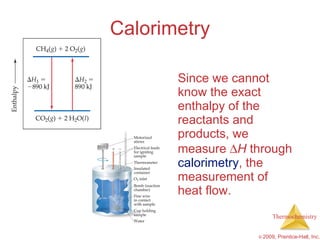
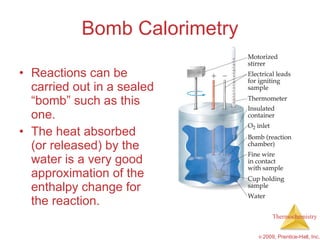
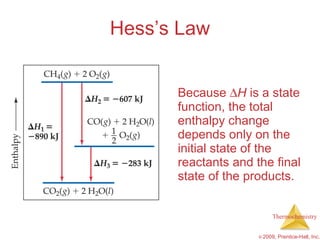
3) Calorimetry allows the measurement of heat and enthalpy changes through experiments on chemical reactions. Hess's law states that the enthalpy change of an overall reaction is the sum of enthalpy changes for individual steps.


















![Hess’s Law Hess’s law states that “[i]f a reaction is carried out in a series of steps, H for the overall reaction will be equal to the sum of the enthalpy changes for the individual steps.”](https://image.slidesharecdn.com/ch05outline-100607165529-phpapp02/85/AP-Chemistry-Chapter-5-Outline-43-320.jpg)
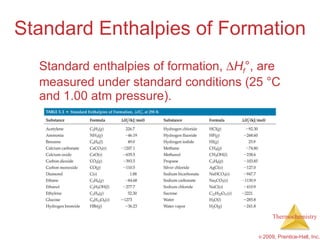
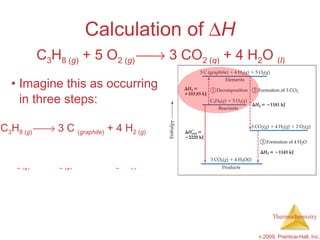
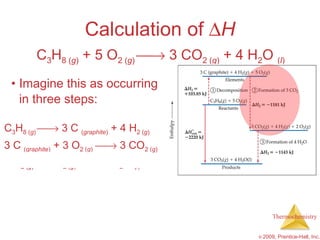
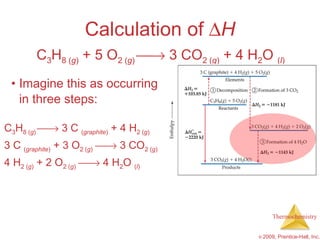
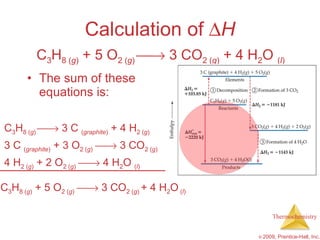
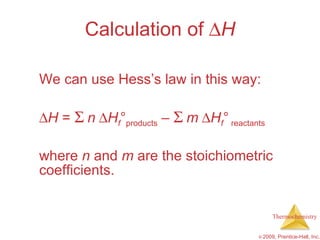
![ H = [3(-393.5 kJ) + 4(-285.8 kJ)] – [1(-103.85 kJ) + 5(0 kJ)] = [(-1180.5 kJ) + (-1143.2 kJ)] – [(-103.85 kJ) + (0 kJ)] = (-2323.7 kJ) – (-103.85 kJ) = -2219.9 kJ Calculation of H C 3 H 8 ( g ) + 5 O 2 ( g ) 3 CO 2 ( g ) + 4 H 2 O ( l )](https://image.slidesharecdn.com/ch05outline-100607165529-phpapp02/85/AP-Chemistry-Chapter-5-Outline-52-320.jpg)