This document discusses acid-base equilibria, including definitions of acids and bases according to Bronsted-Lowry theory. It covers water's behavior as both an acid and a base, pH calculations, strong and weak acids/bases, and polyprotic acids. Key points addressed are: the autoionization of water, conjugate acid-base pairs, the relationship between Ka and Kb, and using Ka or Kb values to determine pH or calculate concentrations.


![• Acids: taste sour and cause dyes to change color.
• Bases: taste bitter and feel soapy.
• Arrhenius: acids increase [H+]; bases increase [OH-] in
solution.
• Arrhenius: acid + base salt + water.
• Problem: the definition confines us to aqueous solution.
Acids and Bases: A Brief Review](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-3-320.jpg)
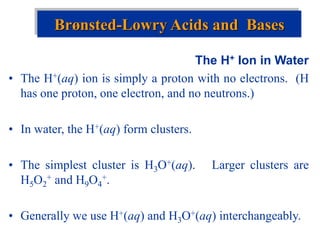
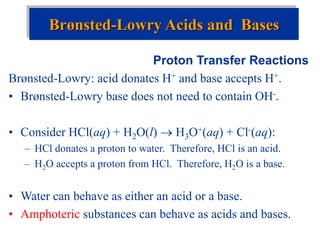
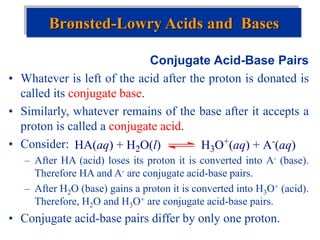

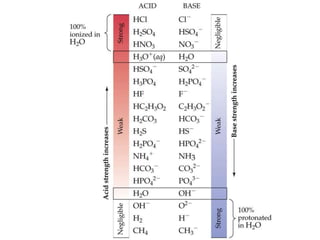
![The Ion Product of Water
• In pure water the following equilibrium is established
• at 25 C
• The above is called the autoionization of water.
The Autoionization of Water
H2O(l) + H2O(l) H3O+(aq) + OH-(aq)
14
-
3
-
3
2
2
2
2
-
3
10
0
.
1
]
OH
][
O
H
[
]
OH
][
O
H
[
]
O
H
[
]
O
H
[
]
OH
][
O
H
[
w
eq
eq
K
K
K](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-9-320.jpg)
![The Autoionization of Water
• [H+] = [OH-] neutral
• [H+] > [OH-] acidic ( [H+] > 1.0x10-7 M )
• [H+] < [OH-] basic ( [H+] < 1.0x10-7 M )](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-10-320.jpg)
![Calculate [H+] in an aqueous solution in which [OH-] is 1.8x10-9 M. Is this an acidic
or basic solution at 25oC?](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-11-320.jpg)
![• In most solutions [H+(aq)] is quite small.
• We define
• In neutral water at 25 C, pH = pOH = 7.00.
• In acidic solutions, [H+] > 1.0 10-7 M , so pH < 7.00.
• In basic solutions, [H+] < 1.0 10-7 M , so pH > 7.00.
• The higher the pH, the lower the pOH, the more basic the
solution.
The pH Scale
]
OH
log[
pOH
]
H
log[
]
O
H
log[
pH -
3
pOH
pH
OH
H
10
]
[
10
]
[](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-12-320.jpg)
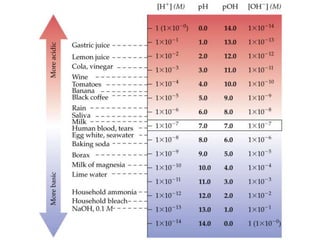
![• Most pH and pOH values fall between 0 and 14.
• There are no theoretical limits on the values of pH or pOH. (e.g.
pH of 2.00 M HCl is -0.301.)
• Number of decimals in the log equals number of sig. figs. in the
original number.
• [Number of decimals in p-scale equals number of sig. figs. in
the concentration value.]
The pH Scale](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-13-320.jpg)
![1. Calculate the pH of an aqueous solution for which (a) [H+] = 1.0x10-7 M ;
(b) [H+] = 1.4x10-3 M; (c) [OH-] = 2.0x10-3 M .
2. An antacid tablet has a pH of 9.18. Calculate the hydrogen ion
concentration.](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-14-320.jpg)
![Other “p” Scales
• In general for a number X,
• For example, pKw = -log Kw.
The pH Scale
X
log
X
p
14
pOH
pH
14
]
OH
log[
]
H
log[
14
]
OH
][
H
[
log
pK
10
0
.
1
]
OH
][
H
[
-
-
w
14
-
w
K](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-16-320.jpg)


![Strong Bases
• Most ionic hydroxides are strong bases (e.g. NaOH,
KOH, and Ca(OH)2).
• Strong bases are strong electrolytes and dissociate
completely in solution.
• The pOH (and hence pH) of a strong base is given by the
initial molarity of the base. Be careful of stoichiometry.
Strong Acids and Bases
1. Calculate pH of 0.029 M NaOH.
2. Calculate [Ca(OH)2] for which the pH is 11.68 .](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-21-320.jpg)
![1. Calculate pH of 0.029 M NaOH.
2. Calculate [Ca(OH)2] for which the pH is 11.68 .](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-22-320.jpg)
![1. Calculate pH of 0.029 M NaOH.
2. Calculate [Ca(OH)2] for which the pH is 11.68 .](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-23-320.jpg)
![• Weak acids are only partially ionized in solution.
• There is a mixture of ions and unionized acid in solution.
• Therefore, weak acids are in equilibrium:
Weak Acids
HA(aq) + H2O(l) H3O+(aq) + A-(aq)
HA(aq) H+
(aq) + A-
(aq)
]
HA
[
]
A
][
O
H
[ -
3
a
K
]
HA
[
]
A
][
H
[ -
a
K](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-24-320.jpg)
![• Ka is the acid dissociation constant.
• Note [H2O] is omitted from the Ka
expression. (H2O is a pure liquid.)
• The larger the Ka the stronger the acid
(i.e. the more ions are present at
equilibrium relative to unionized
molecules).
• If Ka >> 1, then the acid is completely
ionized and the acid is a strong acid.
Weak Acids
Ka Samples](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-25-320.jpg)


![Using Ka to Calculate pH
• Percent ionization is another method to assess acid
strength.
• For the reaction
Weak Acids
HA(aq) + H2O(l) H3O+(aq) + A-(aq)
100
]
HA
[
]
O
H
[
ionization
%
0
3
eqm
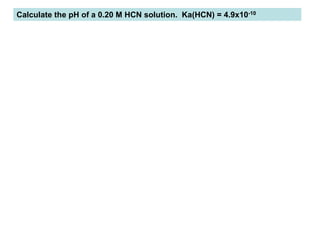
Calculate the pH of a 0.20 M HCN solution. Ka(HCN) = 4.9x10-10](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-28-320.jpg)

![Using Ka to Calculate pH
• Percent ionization relates the equilibrium H+
concentration, [H+]eqm, to the initial HA concentration,
[HA]0.
• The higher percent ionization, the stronger the acid.
• Percent ionization of a weak acid decreases as the
molarity of the solution increases.
• For acetic acid, 0.05 M solution is 2.0 % ionized whereas
a 0.15 M solution is 1.0 % ionized.
Weak Acids](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-31-320.jpg)
![100
]
[
%
2
1
x
HA
Ka
I
o
](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-32-320.jpg)
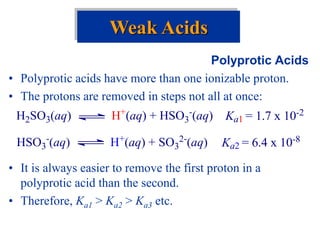
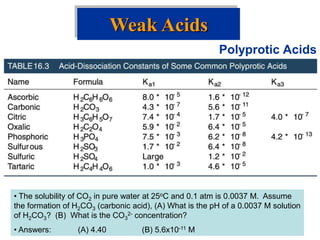
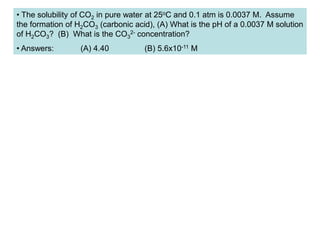
![• Weak bases remove protons from substances.
• There is an equilibrium between the base and the
resulting ions:
• Example:
• The base dissociation constant, Kb , is defined as
Weak Bases
Weak base + H2O conjugate acid + OH-
NH3(aq) + H2O(l) NH4
+(aq) + OH-(aq)
]
NH
[
]
OH
][
NH
[
3
-
4
b
K](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-37-320.jpg)
![Types of Weak Bases
• Bases generally have lone pairs or negative charges in order to
attack protons.
• Most neutral weak bases contain nitrogen.
• Amines are related to ammonia and have one or more N-H bonds
replaced with N-C bonds (e.g., CH3NH2 is methylamine).
• Anions of weak acids are also weak bases. Example: OCl- is the
conjugate base of HOCl (weak acid):
Weak Bases
ClO-(aq) + H2O(l) HClO(aq) + OH-(aq) Kb = 3.3 x 10-7
• Calculate [OH-] and pH of a 0.15 M solution of NH3 . (Kb = 1.8x10-5)](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-38-320.jpg)

![• Calculate [OH-] and pH of a 0.15 M solution of NH3 . (Kb = 1.8x10-5)
Ans: pH = 11.22
Solution](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-40-320.jpg)

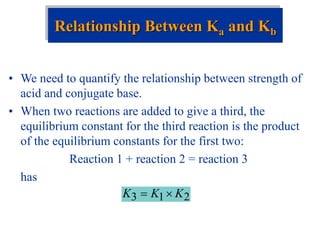
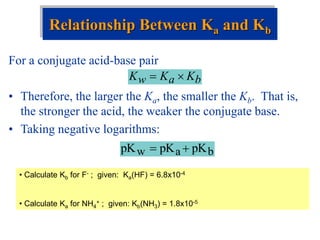


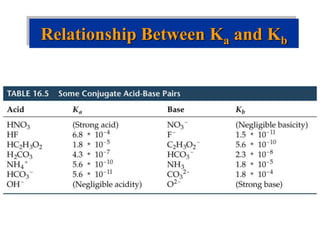


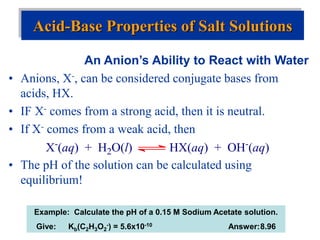




![Factors that Affect Acid Strength
Consider H-X. For this substance to be an acid we need:
• H-X bond to be polar with H+ and X- (if X is a metal
then the bond polarity is H-, X+ and the substance is a
base), [ i.e. Large Electronegativity ]
• the H-X bond must be weak enough to be broken, [ i.e.
Small Bond Strength ]
• the conjugate base, X-, must be stable. [ i.e. Weak base ]
Acid-Base Behavior and Chemical Structure](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-55-320.jpg)
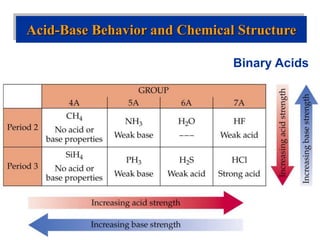
![Binary Acids
• Acid strength increases across a period [ EN ] and down
a group [ bond strength, size ]
• Conversely, base strength decreases across a period and
down a group.
• HF is a weak acid because the bond energy is high.
• The electronegativity difference between C and H is so
small that the C-H bond is non-polar and CH4 is neither
an acid nor a base.
Acid-Base Behavior and Chemical Structure](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-56-320.jpg)
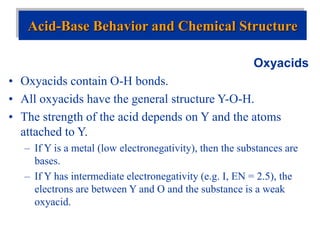
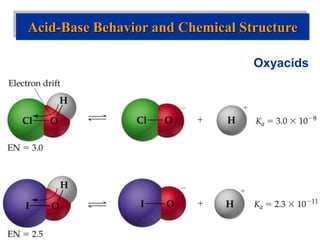
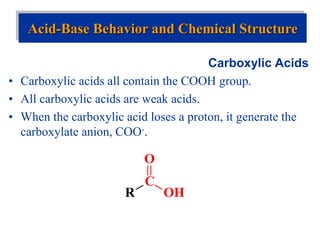



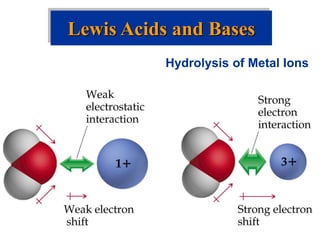
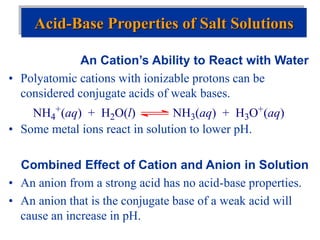
![Acid-Base Equilibria
Arrhenius
Definition
Autoionization
of Water
Bronsted-Lowry
Acids-Bases
pH Scale
Weak Acids Weak Bases
Strong
Acids/Bases
Chemical
Structure
Salt Solutions
Lewis
Acids/Bases
14
-
10
0
.
1
]
OH
][
H
[
w
K ]
H
log[
pH
14
pOH
pH
]
HA
[
]
A
][
H
[ -
a
K
100
]
HA
[
]
H
[
I
%
0
eqm
b
a
w K
K
K
Conjugate Acid-Base Pairs](https://image.slidesharecdn.com/acid-baseequilibria-220912230333-9671a5d0/85/Acid-Base-Equilibria-ppt-65-320.jpg)