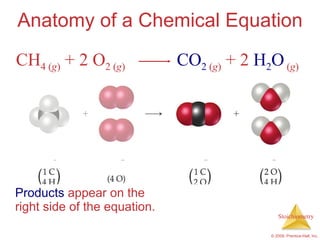
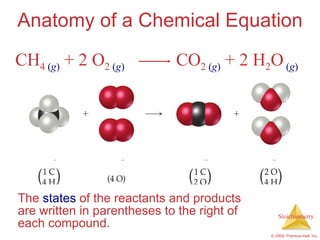
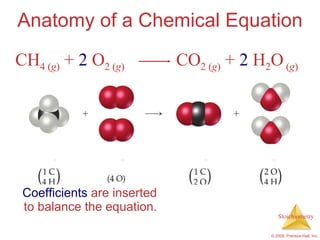
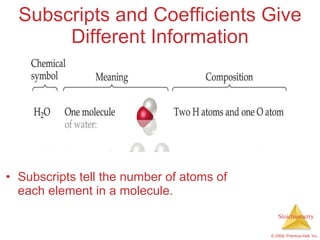
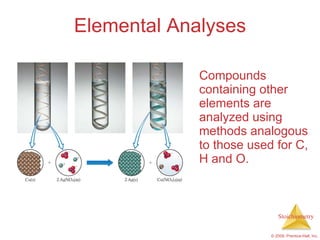
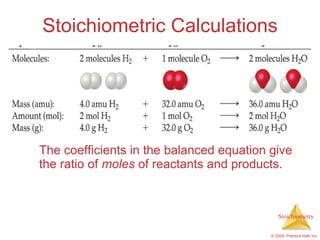
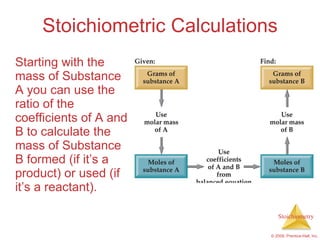
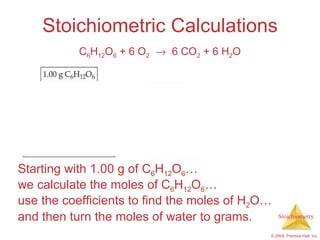
This document summarizes key concepts in stoichiometry including:
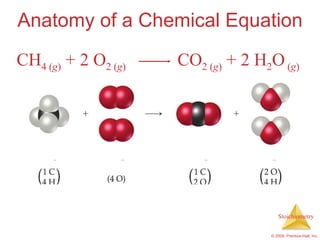
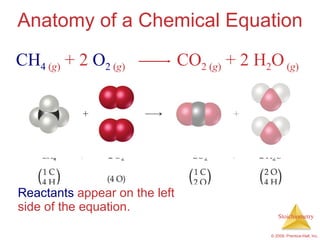
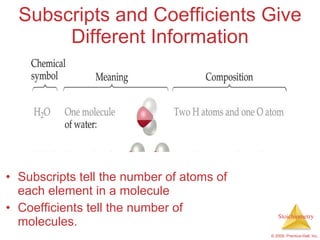
1) Chemical equations represent chemical reactions and include reactants, products, and coefficients to balance the equation.
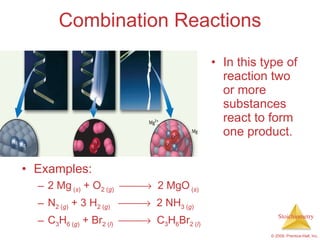
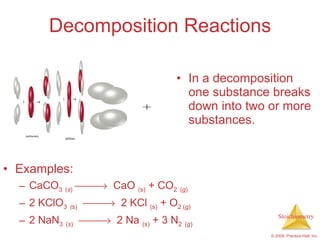
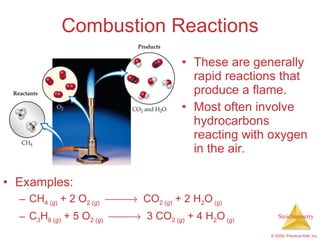
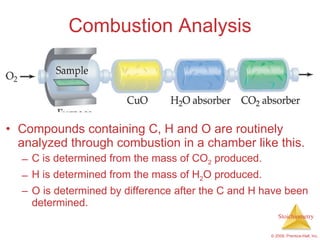
2) Reaction types include combination, decomposition, and combustion reactions.
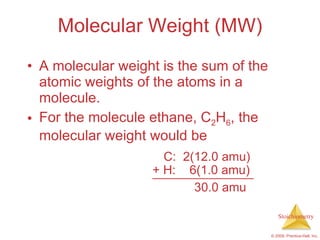
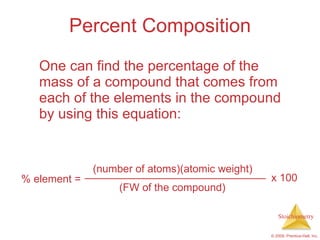
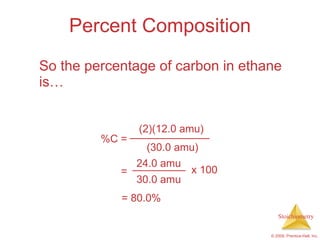
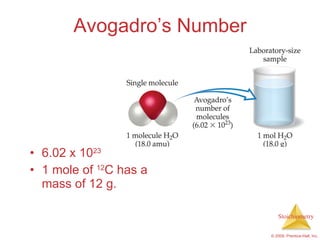
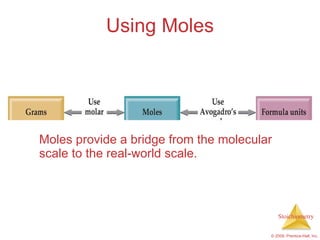
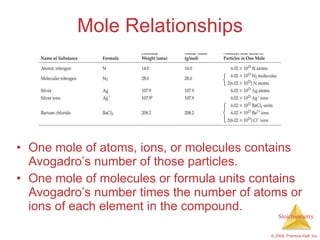
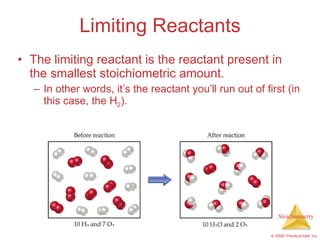
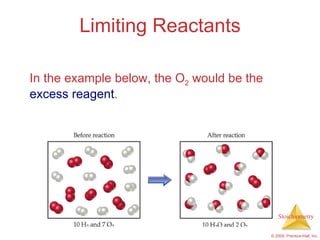
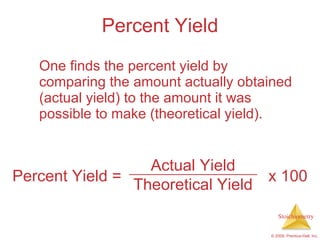
3) Formula weights, molecular weights, moles, molar mass, and limiting reactants are important concepts for solving stoichiometry problems quantitatively.
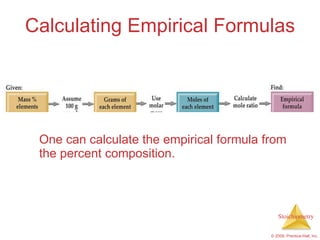
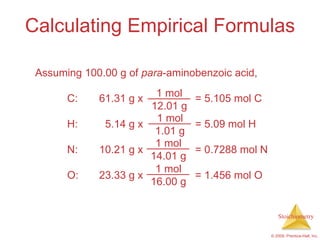
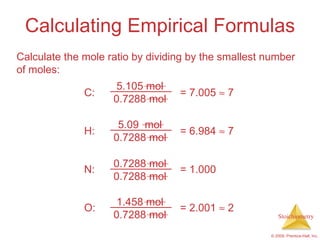
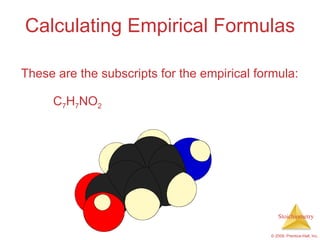
4) Empirical formulas can be calculated from elemental composition data using mole ratios.