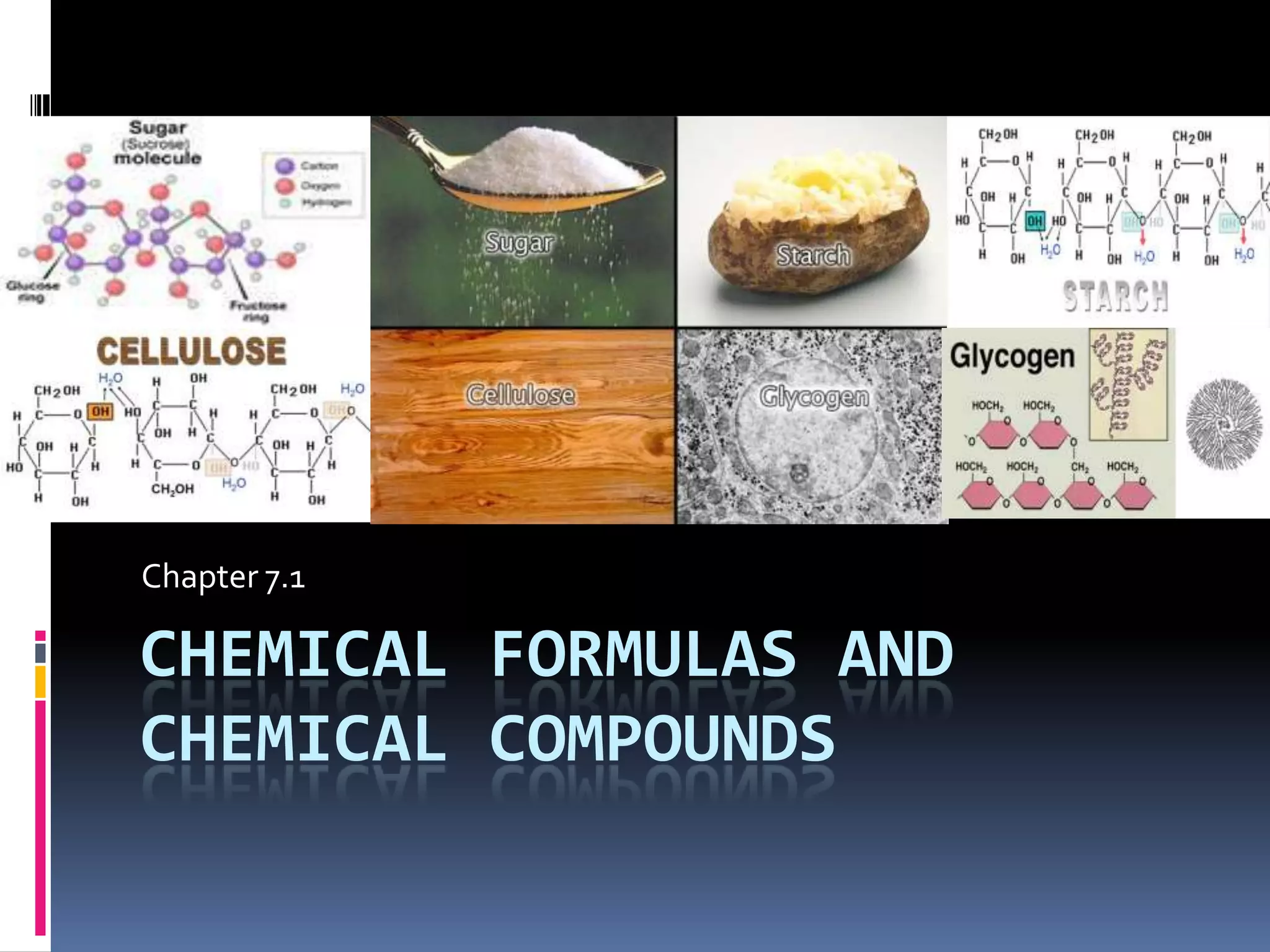
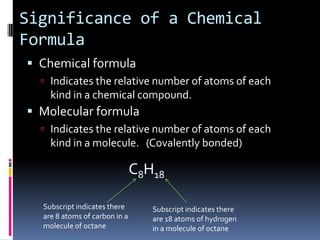
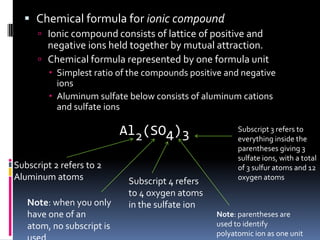
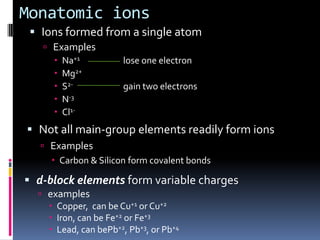
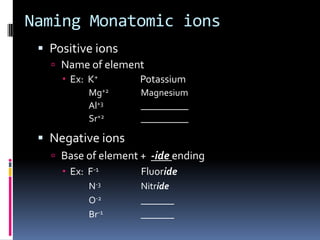
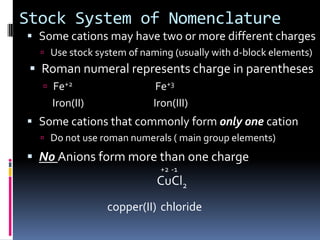
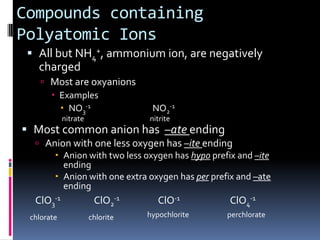
Chemical formulas indicate the relative number and type of atoms in a chemical compound or molecule. A chemical formula for an ionic compound represents one formula unit and uses the simplest whole number ratio of ions present. Binary ionic compounds are composed of two elements and the total positive charges must equal the total negative charges. Molecular compounds use a prefix system to indicate the number of atoms of the less electronegative element present. Polyatomic ions are named as units within chemical formulas.