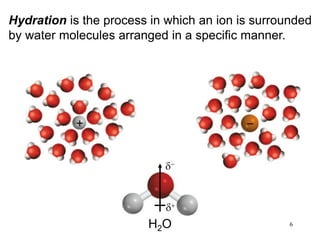
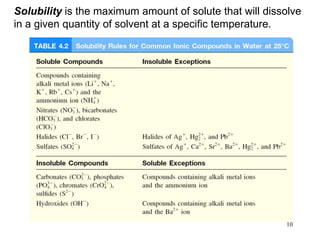
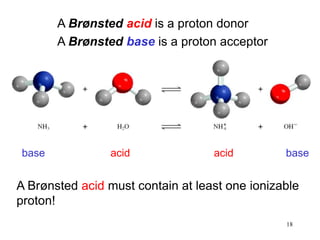
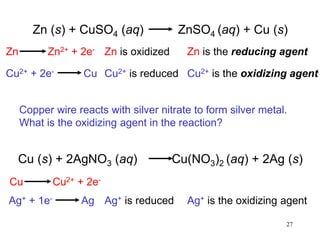
1) A solution is a homogeneous mixture of two or more substances, where the solute is present in smaller amounts than the solvent.
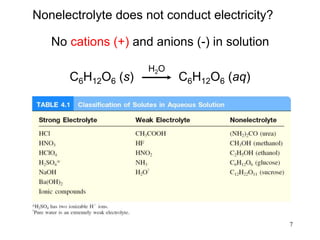
2) An electrolyte is a substance that, when dissolved in water, allows the solution to conduct electricity through the formation of ions, while a nonelectrolyte does not form ions and the solution cannot conduct electricity.
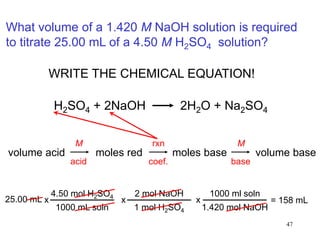
3) Titrations involve slowly adding a solution of known concentration to another solution of unknown concentration until the chemical reaction between them is complete, as indicated by an indicator, and can be used to determine concentrations in acid-base and redox reactions.