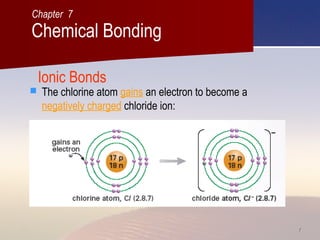
The document discusses different types of chemical bonds and macromolecular structures. It explains that ionic bonds form between metals and non-metals via the transfer of electrons, giving ionic compounds high melting points and the ability to conduct electricity when molten or dissolved. Covalent bonds form between non-metals by the sharing of electrons, resulting in covalent compounds having low melting points and the inability to conduct electricity. Some covalent substances exist as macromolecules or giant molecular structures like diamond and graphite. These have very high melting points and different properties compared to simple molecules. Metallic bonding is also described, involving positive metal ions in a "sea of electrons" giving metals properties like malleability and high conductivity